Rat Anti-Human Alpha 2 (IV) Antibody, Clone H25, Biotinylated
- SKU:
- 445-70751
- Size:
- 1 mg/ml x 0.1 ml
- Shipping:
- Gel Packs
- Storage:
- - 20C
Description
Rat Anti-Human Alpha 2 (IV) Antibody, Clone H25, Biotinylated - Cat Number: 70751 From Chondrex.
Research Field: Nephritis, ECM
Clonality: Monoclonal
Cross-Reactivity: Human alpha 2 (IV)
Host Origin: Rat
Applications: IHC, Immunoblotting
Isotype: IgG1
Detection Range: N/A
Sample Type: N/A
Concentration: 1 mg/ml
Immunogen: QEAIQPGCG
DESCRIPTION: Rat IgG1 monoclonal antibody against the human non-collagenase domain 1 (NC1) of the alpha
2 chain of type IV collagen (alpha 2 (IV)), clone H25, biotinylated.
Clone H25 recognizes alpha 2 (IV) (Epitope: QEAIQPGCG)
APPLICATION: Use for immunohistochemistry and immunoblotting
QUANTITY: 0.1 ml, 1 mg/ml
FORM: Solution in 0.05M Phosphate Buffered Saline, pH 7.4
SOURCE: Rat
REACTIVITY: Reacts to human alpha 2 (IV) NC1
IMMUNOGEN: Peptide: CDTDVKRAVGGDRQEAIQPG
PURITY: Purified by Protein G chromatography
SUBTYPE: Rat IgG1
USAGE: Dilute the antibody in buffer containing 2% heterologous serum, such as normal goat serum, to
prevent non-specific reactions in tissue staining or immunoblotting.
STORAGE: -20°C
STABILITY: 1 year
NOTES: N/A
REFERENCES: Y. Sad, M. Kagawa, Y. Kishiro, K. Sugihara, I. Naito, J. Seyer, M. Sugimoto, T. Oohashi, Y.
Ninomiya, Histochem Cell Biol 104:267-75 (1995).
I. Naito, S. Kawai, S. Nomura, Y. Sado, G. Osawa, Kidney Int 50:304-11 (1996).
M. Kagawa, Y. Kishiro, I. Naito, T. Nemoto, H. Nakanishi, Y. Ninomiya, Y. Sado. Nephrol Dial
Transplant 6 1238-41 (1997)
INTRODUCTION
In the kidney, the glomerular basement membranes (GBM) contain type-IV collagen which consists of trimers of α3, α4, and α5 chains (α3(IV), α4(IV), and α5(IV), respectively) and heterotrimers of α1(IV) and α2(IV) chains (1). Genetic mutation of the α5(IV) chain, which is regulated by the X chromosome, disrupts the formation of the α3-4-5 networks and leads to abnormal GBM development. This structural defect causes nephritis known as X-linked Alport syndrome. Alport syndrome can be diagnosed by pathogenesis tests which detect the type IV collagen mutation by immunostaining the collagen chains and can be further evaluated by kidney function tests which determine urinary protein levels (2).
Additionally, skin epidermal basement membranes (EBM) similarly contain type-IV collagen composed of heterotrimers of α1(IV) and α2(IV), and heterotrimers of α5(IV) and α6(IV). Therefore, determining α5(IV) chain mutations in skin biopsy sections by immunostaining is a useful method for diagnosing Alport syndrome due to the minimally invasive method of sample preparation (3).
Chondrex, Inc. provides the two epitope-defined biotinylated rat monoclonal antibodies (mAbs) against the α2(IV) chain (Cat # 70751, H25) and the α5(IV) chain (Cat # 70781, H53). Immunostaining using the anti-α5(IV) mAb in Alport syndrome patient samples shows abnormal distributions and discontinuous patterns of type IV collagen due to the mutation of the collagen chains in the GBM while immunostaining with the anti-α2(IV) mAb shows normal structural patterns (4, 5). The following immunostaining protocol using these mAbs may be helpful for studying the mechanism and pathogenesis of Alport syndrome (1, 6).
PROCEDURE
A. Cryostat Sections
An immunostaining method for α2(IV) and α5(IV) on cryostat sections by biotinylated rat monoclonal antibodies (H25: Cat # 70751 and H53: Cat # 70781). The following protocol may need to be optimized depending on sample type.
1. Cut two 3 μm thick cryostat sections, air dry, and fix in acetone for 10 minutes at room temperature.
2. Wash the two slides in 0.05M phosphate saline buffer, pH 7.4 for 5 minutes.
3. Block the two slides with 1% casein in 0.05M phosphate saline buffer, pH 7.4 for 30 minutes.
4. Incubate the two slides with the following biotinylated monoclonal antibodies for 1 hour: H25 (diluted 1:25 in 1% casein in 0.05M phosphate saline buffer, pH 7.4) H53 (diluted 1:25 in 1% casein in 0.05M phosphate saline buffer, pH 7.4).
5. Wash the two slides in 0.05M phosphate saline buffer, pH 7.4 for 10 minutes.
6. Incubate the two tissue slides with FITC-labeled Avidin in 1% casein in 0.05M phosphate saline buffer, pH 7.4 for 1 hour.
7. Wash in 0.05M phosphate saline buffer, pH 7.4 for 10 minutes and add mounting media containing p-Phenylenediamine to delay fluorescence quenching.
B. Paraffin-Embedded Samples
An immunostaining method for α2 (IV) NC1 and α5(IV) on formalin-fixed, paraffin-embedded tissue sections by biotinylated rat monoclonal antibodies (H25: Cat # 70751 and H53: Cat # 70781). The following protocol may need to be optimized depending on sample type.
1. Cut two 3-4 μm thick formalin-fixed, paraffin-embedded tissue sections, deparaffinize, and rehydrate.
2. Immerse the sections with 0.2M HCl, pH 0.9 and heat in a small autoclave at 110-127°C for 6 minutes. (Alternatively, boil sections in citrate buffer, pH 2.0 in a microwave oven at 750W for 8 minutes followed by 350W for 15 minutes)
3. Rinse two slides in distilled water for 2 minutes.
4. Block the two slides with 1% casein in 0.05M phosphate saline buffer, pH 7.4 for 30 minutes.
5. Incubate the two slides with the following biotinylated monoclonal antibodies for 1 hour: H25 (diluted 1:50 in 1% casein in 0.05M phosphate saline buffer, pH 7.4) H53 (diluted 1:50 in 1% casein in 0.05M phosphate saline buffer, pH 7.4).
6. Wash the two slides in 0.05M phosphate saline buffer, pH 7.4 for 10 minutes.
7. Incubate the two tissue slides with FITC-labelled Avidin in 1% casein in 0.05M phosphate saline buffer, pH 7.4 for 1 hour.
8. Wash in 0.05M phosphate saline buffer, pH 7.4 for 10 minutes and add mounting media containing p-Phenylenediamine to delay fluorescence quenching.
INTERPRETING STAIN RESULTS
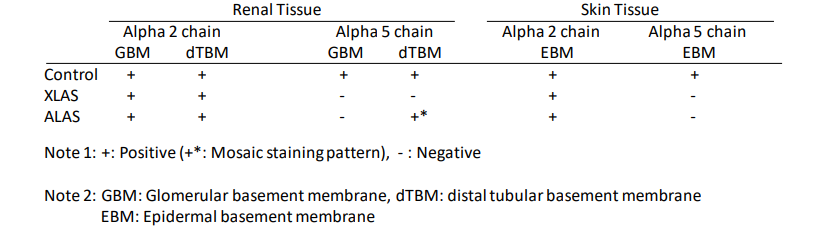
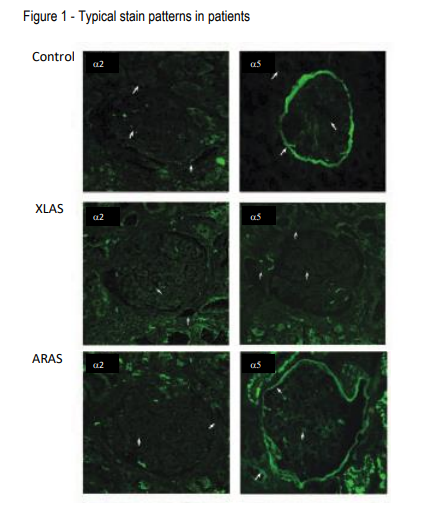
In the kidney biopsy samples from normal patients, H25 and H53 linearly stain the glomerular basement membrane (GBM), the Bowman’s capsules (BC), and the distal tubular basement membranes (dTBM) (Figure 1 Top). In males with X-linked Alport syndrome (XLAS), H25 stains the tissue same as in normal patients, however, H53 fails to stain the GBM, BC, and dTBM (Figure 1 Middle). In females with XLAS, H53 discontinuously stains the GBM, but not the BC and dTBM. In autosomal recessive Alport syndrome (ARAS), although H25 stains the GBM, BC, and dTBM, H53 stains with a mosaic pattern in the BC and dTBM (Figure 1 bottom). Table 1 and Figure 1 show typical staining patterns in the three groups. Therefore, these two monoclonal antibodies work to distinguish XLAS and ARAS by immunohistochemical
analysis of renal biopsies.
Using skin biopsy samples, H25 stains the epidermal basement membrane (EBM) in all patients, however, H53 stains the EBM only in normal patients, and failed to stain the EBM in XLAS and ARAS patients (Table 1).
NOTE: the staining results may vary due to sample preparation. Chondrex, Inc. recommends establishing your own control references.
Table1. Typical staining results using anti-type IV collagen antibodies in patients.
REFERENCES
1. I. Naito, S. Nomura, S. Inoue, M. Kagawa, S. Kawai, et al., Normal Distribution of Collagen IV in Renal Basement Membranes in Epstein's Syndrome. J Clin Pathol 50, 919-22 (1997).
2. C. Kashtan, A. F. Michael, Alport syndrome. Kidney International. 50, 1445–1463 (1996).
3. E. Lagona, L. Tsartsali, S. Kostaridou, A. Skiathitou, E. Georgaki, F. Sotsiou, et al., Skin Biopsy for the Diagnosis of Alport Syndrome. Hippokratia 12, 116-8 (2008).
4. M. Kagawa, Y. Kishiro, I. Naito, T. Nemoto, H. Nakanishi, et al., Epitope-defined Monoclonal Antibodies Against type-IV Collagen for Diagnosis of Alport's Syndrome. Nephrol Dial Transplant 12, 1238-41 (1997).
5. D. Borza, O. Bondar, Y. Ninomiya, Y. Sado, I. Naito, et al., The NC1 Domain of Collagen IV Encodes a Novel Network Composed of the Alpha 1, Alpha 2, Alpha 5, and Alpha 6 Chains in Smooth Muscle Basement Membranes. J Biol Chem 276, 28532-40 (2001).
6. K. Yoshioka, S. Hino, T. Takemura, S. Maki, J. Wieslander, et al., Type IV Collagen Alpha 5 Chain. Normal Distribution and Abnormalities in X-linked Alport Syndrome Revealed by Monoclonal Antibody. Am J Pathol 144, 986-96 (1994).
7. Kashtan, Alport Syndromes: Phenotypic Heterogeneity of Progressive Hereditary Nephritis. Pediatr Nephrol 14, 502-12 (2000).






