LaunchWorks Viral Transport Media (VTM)
The FDA has issued Emergency Use Authorizations (EUA) for numerous molecular and antigen tests for detection of SARS-CoV-2 (COVID-19). Our Viral Transport Media is listed per FDA guidance, “Enforcement Policy for Viral Transport Media during the Coronavirus 2019 (COVID-19) Public Health Emergency.” The VTM is manufactured in the LaunchWorks CDMO facility which is FDA registered, cGMP compliant, and ISO 13485:2016 certified.
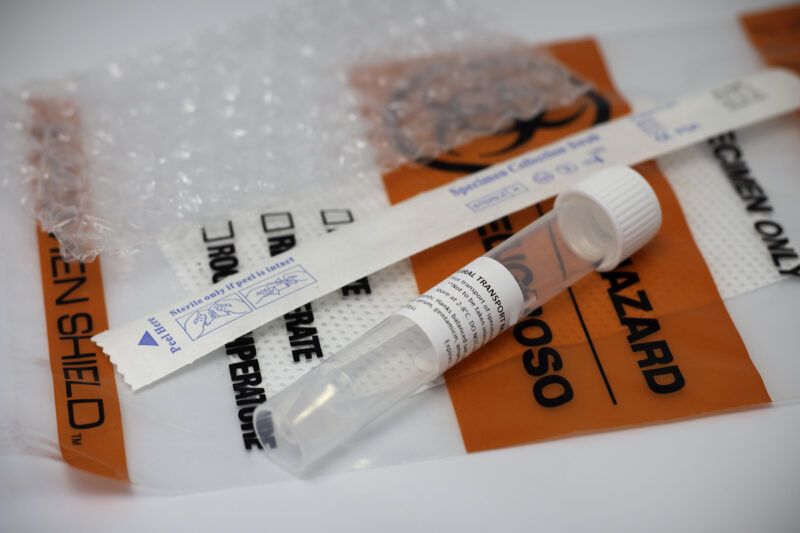
VTM Specimen Kit:
- 1 tube with 3 mLs of sterile LaunchWorks VTM
- Biohazard bag - tube labeled per CDC instructions
- Absorbent pad
Options:
- Nylon-flocked NP swab
- Barcode or QP-coded
labels (up to 3)
- Patient Card
- UN 3373 Return Box
Swab Specifications:
- Naseopharyngeal swab
- Individually packaged
- Nylon flocked
- 155mm, 80 mm breakpoint
- FDA-registered Manufacturer
- Ethylene Oxide Sterlization
- 2 year shelf-life
- CE-marked
Launchworks VTM Recipe:
- Fetal Bovine Serum (sterile, heat
inactivated, USDA approved)
- Hanks Balanced Salt Solution
(no phenol red)
- Gentamicin
- Amphotericin B
- Physiological pH range
Media Specifications
- Microbial Sterility Tested
- DNAse and Protease free Tested
Viral Transport Media (VTM)
| Product | VTM Filled Tubes | VTM Filled Tubes | VTM Filled Tubes | VTM Filled Tubes | VTM Filled Tubes |
| Part Number | LWBVTMCT03 | LWBVTMCB1L | LWBVTMCBK | LWBVTMCK01 | LWBVTMCK01 |
| Description | 3 mL of VTM in IATA RATED 12 mL tube | 1L Bottles | 100 labeled tubes & swabs, biohazard bags in bulk | Labeled tube in biohazard bag | Any combinination of additional parts |
| Configuration | Case of 50 or 500 | One (1) bottle or case of six (6) | Case of 100 | Cases of 100 or greater | Cases of 100 or greater |
| Options | Patient Label | None | None | None | See Belo |
