Description
Hektoen Enteric (HE) Agar | NCM0006
Intended Use
Hektoen Enteric Agar is used for the isolation and differentiation of enteric pathogens. Hektoen Enteric Agar is not intended for use in the diagnosis of disease or other conditions in humans.
Description
Hektoen Enteric Agar was developed in 1967 by King and Metzger. Compared to other enteric differentiating media commonly used, Hektoen Enteric Agar increased the isolation rate of Salmonella spp. and Shigella spp. This was accomplished by increasing the carbohydrate and peptone content of the medium in order to counteract the inhibitory effects of bile salts and indicators. King and Metzger formulated a medium that slightly inhibited growth of Salmonella and Shigella, while inhibiting Grampositive microorganisms.
Principles of the Procedure
Enzymatic Digest of Animal Tissue provides nitrogen, carbon, and amino acids required for organism growth. Yeast Extract is a vitamin source. Bile Salts Mixture and Acid Fuchsin inhibit Gram-positive organisms. Lactose, Sucrose, and Salicin are fermentable carbohydrates. Sodium Chloride maintains the osmotic balance of the medium. Ferric Ammonium Citrate, a source of iron, allows the detection of hydrogen sulfide (H2S) produced from Sodium Thiosulfate. H2S-positive colonies have black centers.Bromothymol Blue is added as the pH indicator. Agar is the solidifying agent.
Typical Formulation
Enzymatic Digest of Animal Tissue 12.0 g/L
Yeast Extract 3.0 g/L
Bile Salts Mixture 9.0 g/L
Lactose 12.0 g/L
Sucrose 12.0 g/L
Salicin 2.0 g/L
Sodium Chloride 5.0 g/L
Sodium Thiosulfate 5.0 g/L
Ferric Ammonium Citrate 1.5 g/L
Bromothymol Blue 0.065 g/L
Acid Fuchsin 0.1 g/L
Agar 13.5 g/L
pH: 7.5 ± 0.2 at 25°C
Formula may be adjusted and/or supplemented as required to meet performance specifications.
Precaution
Refer to SDS
Preparation
1. Suspend 75 g of the medium in one liter of purified water.
2. Heat with frequent agitation and boil for one minute to completely dissolve the medium.
3. DO NOT AUTOCLAVE.
Test Procedure
For isolation and identification of pathogenic Enterobacteriaceae refer to appropriate references.
Quality Control Specifications
Dehydrated Appearance: Powder is homogeneous, free flowing, and light greenish beige.
Prepared Appearance: Prepared medium is trace to slightly hazy and light to dark green.
Expected Cultural Response: Cultural response on Hektoen Enteric Agar at 35 ± 2°C after 18 - 24 hours incubation.
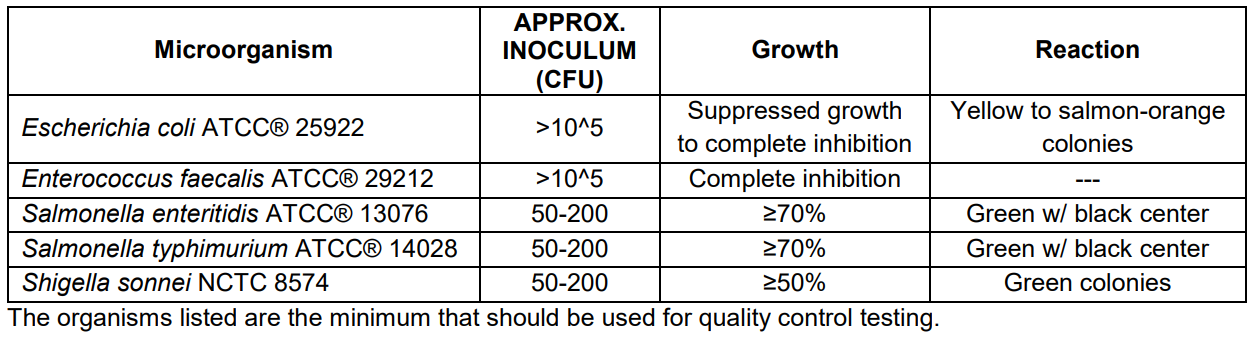
Results
Refer to appropriate references and procedures for results.
Expiration
Refer to expiration date stamped on the container. The dehydrated medium should be discarded if not
free flowing, or if appearance has changed from the original color. Expiry applies to medium in its intact
container when stored as directed.
Limitations of the Procedure
1. Do not autoclave medium because excessive heat may alter ingredients.
2. Proteus spp. may resemble salmonellae or shigellae. Further testing should be conducted to confirm the presumptive identification or organisms isolated on this medium.
3. Due to nutritional variation, some strains may be encountered that grow poorly or fail to grow on this medium.
Storage
Store dehydrated culture media at 2 – 30°C away from direct sunlight. Once opened and recapped, place the container in a low humidity environment at the same storage temperature. Protect from moisture and light by keeping container tightly closed.






