Description
NATtrol™ Respiratory Syncytial Virus Type A Stock* is formulated with purified, intact viral particles that have been chemically modified to render them non-infectious and refrigerator stable. Each vial contains 1.0 mL of NATtrol™ Respiratory Syncytial Virus Type A stock in a
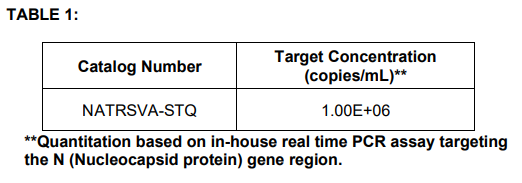
proprietary matrix at the target concentration listed in Table 1.
INTENDED USE:
• NATtrol™ Respiratory Syncytial Virus Type A stock is designed to evaluate the performance of nucleic acid tests for determination of the presence of Respiratory Syncytial
Virus A nucleic acid. NATtrol™ Respiratory Syncytial Virus A stock enables laboratories to monitor test variation, lot-tolot test kit performance, operator variation, and can provide assistance in identifying random or systemic error.
WARNINGS AND PRECAUTIONS:
• NATtrol™ inactivation was carried out on Respiratory Syncytial Virus Type A stock used to formulate the product. The inactivation was verified in a standard microbiological
growth protocol.
• This product contains inactivated microorganisms and materials of human and animal origin. Safe practices suggest that the controls be considered potentially infectious and to use Universal Precautions when handling.
• Refer to CDC guidelines and local regulations for handling and disposal.
• The matrix used in the manufacture of this product is treated with 0.09% sodium azide. It was manufactured from Human Serum Albumin that has been tested and found to be nonreactive at the donor level for HIV-1/HIV-2 Antibody, HBsAg and HCV Antibody by FDA licensed donor screening test methods. All materials are also tested for HIV-1 and HCV by
FDA approved Nucleic Acid Test (NAT) methods.
• Heat inactivated Fetal Bovine Serum used in the manufacture of this product meets applicable USDA requirements for abattoir sourced animals, traceability and
country of origin. The materials were collected at USDA licensed establishments or legally imported from countries recognized by the USDA as negligible or controlled for risk for Bovine Spongiform Encephalopathy (BSE) and other exotic disease agents. Donor animals were inspected ante and post mortem at the abattoir as required by the USDA.
• Do not use past the expiration date on the label.
• To avoid cross-contamination, use separate pipette tips for all materials.
RECONDED STORAGE:
• NATtrol™ Respiratory Syncytial Virus Type A Stock should be stored at 2-8°C.
INSTRUCTIONS FOR USE:
• Mix vial vigorously for at least 5 secs.
• Process according to manufacturer’s instructions for sample to result assays.
• Extract nucleic acid prior to use in downstream assays that are not sample to result.
LIMITATION:
• FOR RESEARCH USE ONLY. NOT FOR USE IN
DIAGNOSTIC PROCEDURES
• Quality control materials should be used in accordance with local, state, federal, and accreditation requirements.
• This product is not intended to replace the manufacturer’s controls provided with the assay.
EXPECTED RESULTS:
• Each laboratory must evaluate the product and establish their own acceptance criteria.
• The table shown below is for informational purposes only
3 Reviews
-
good
Well-mannered employees with reliable service are the hallmarks of this company
-
magnífico
Gentaur cumple con sus promesas. Envían todos sus productos a tiempo sin demoras.
-
Very fast delivery
Este producto no está disponible en ningún otro lugar; He buscado mucho en Internet, pero solo está disponible aquí. Cuando lo recibí, lo entregué rápidamente por mensajería a un precio asequible, así que le di cinco estrellas.