Deciphering the Mechanisms of Dengue Virus-Like Particles (VLPs) for Advancing Vaccine Development: A Comprehensive Analysis
Dengue fever remains a significant global health concern, with millions of infections reported annually. Traditional vaccine development for dengue has encountered challenges, including the need for balanced immune responses against all four dengue virus serotypes. However, recent advancements in biotechnology have introduced a promising solution: Dengue Virus-Like Particles (VLPs).
What are Dengue VLPs?
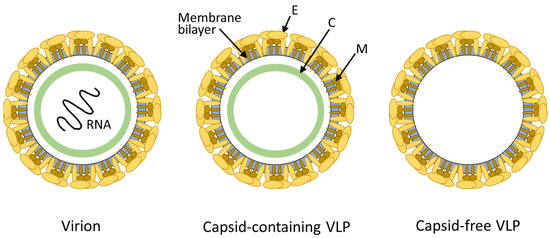
Dengue VLPs are non-infectious, self-assembled structures that mimic the outer shell of the dengue virus. They lack the viral genetic material necessary for replication, making them safe for use in vaccines. Despite their non-infectious nature, Dengue VLPs possess the same surface proteins (envelope and membrane proteins) as the actual virus, enabling them to induce a robust immune response.
How are Dengue VLPs Produced?
The production of Dengue VLPs typically involves recombinant DNA technology. Scientists introduce the genes encoding the viral envelope and membrane proteins into a suitable expression system, such as bacteria, yeast, or mammalian cells. These cells then produce the viral proteins, which self-assemble into VLPs. Purification techniques are employed to isolate and concentrate the VLPs for further characterization and vaccine formulation.
Advantages of Dengue VLPs:
- Safety: Since Dengue VLPs lack the viral genetic material, they cannot cause infection or replicate within the host. This feature eliminates the risk of vaccine-induced disease.
- Immunogenicity: Dengue VLPs closely resemble the authentic virus, eliciting a strong immune response. They stimulate both humoral (antibody-mediated) and cellular immune responses, crucial for providing protection against dengue infection.
- Cross-Reactivity: Dengue VLPs can induce immune responses against multiple dengue virus serotypes simultaneously. This cross-reactivity is essential for developing vaccines capable of conferring broad protection against all four dengue serotypes.
- Scalability: The production of Dengue VLPs can be scaled up to meet vaccine demand, offering a viable solution for large-scale immunization campaigns.
Potential Challenges and Future Directions:
Despite the promise of Dengue VLPs, several challenges remain. These include optimizing vaccine formulations to enhance stability and immunogenicity, addressing potential concerns regarding long-term safety, and ensuring affordability and accessibility, particularly in resource-limited settings.
Future research efforts are focused on fine-tuning Dengue VLP vaccines, exploring novel adjuvants to boost immune responses, and evaluating their efficacy in clinical trials. Additionally, ongoing surveillance and monitoring are necessary to assess vaccine safety and effectiveness post-licensure.
In conclusion, Dengue VLPs represent a groundbreaking approach to dengue vaccine development, offering a safe, immunogenic, and potentially cost-effective solution to combat this debilitating disease. Continued research and collaboration are essential to realizing the full potential of Dengue VLP vaccines and ultimately reducing the global burden of dengue fever.
Recent Posts
-
Nicholas E. Navin’s Keynote on Breast Cancer at SCSOmics 2025 (with Lieven in Attendance)
At SCSOmics 2025, held earlier this year, Nicholas E. Navin, PhD, delivered a keynote address that d …17th Sep 2025 -
Reflections on Our Experience at the Vatican Longevity Summit
On March 24, 2025, the Vatican opened its doors to a remarkable gathering of scientists, ethicists, …25th Mar 2025 -
Gentaur : The Leading Distributor of Applied Biological Materials (ABM) in Belgium and Europe
Driving Scientific Innovation with ABM's Cutting-Edge Products Gentaur has long been the trusted dis …19th Mar 2025