Description
Syphilis Mixed Titer Panel - 15 X 1mL
Donor Demographics: The syphilis positive donor plasmas used for this panel were obtained under informed consent and collected
in US FDA licensed plasma centers. Donors were untreated and asymptomatic at the time of collection.
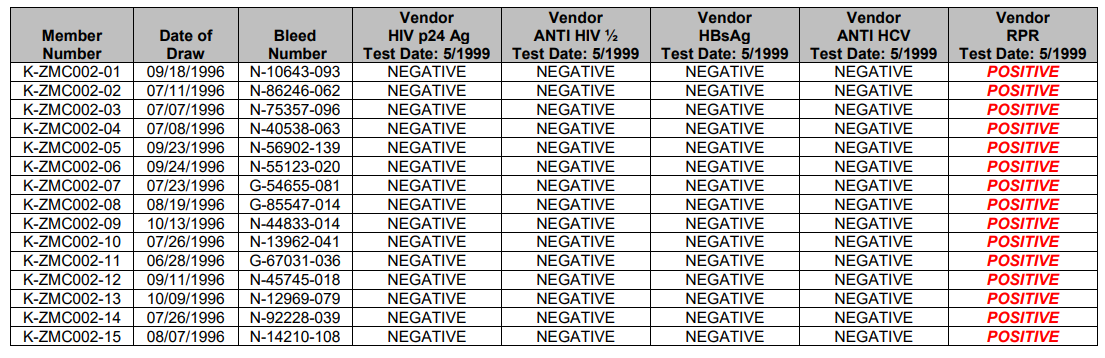
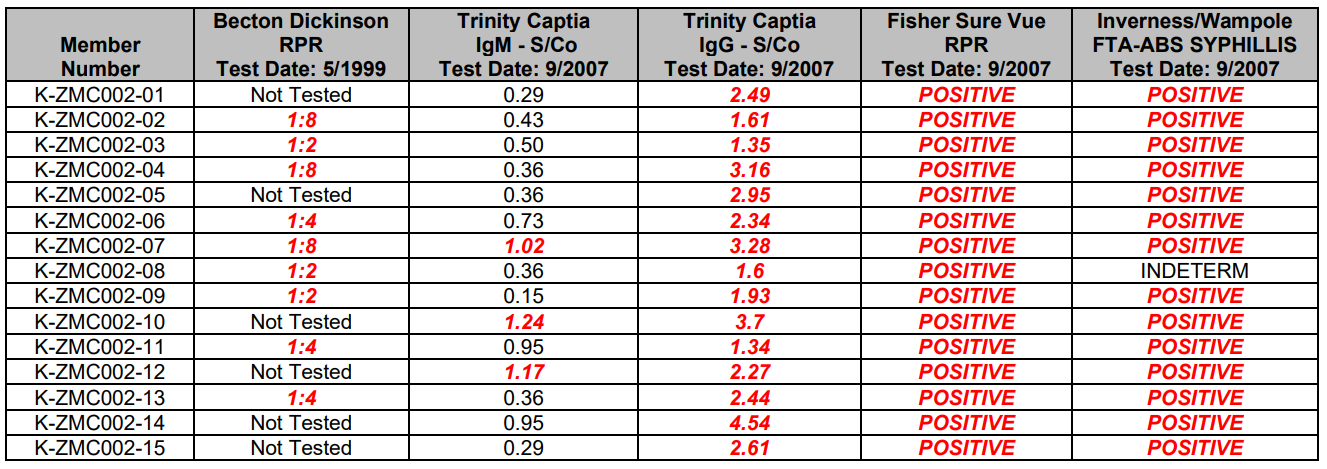
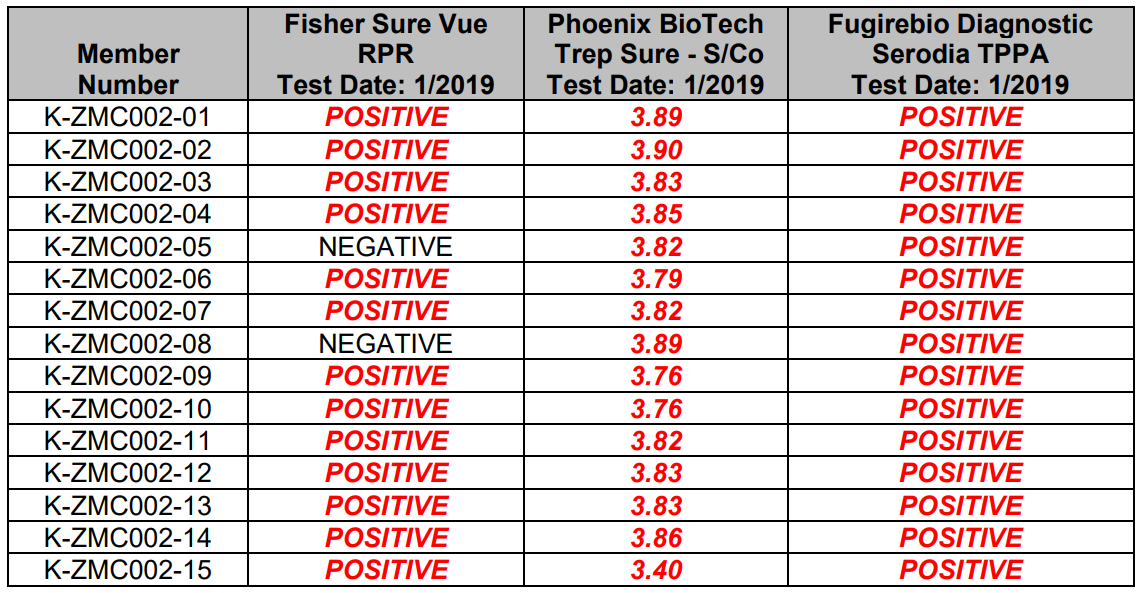
This data is intended to be representative of typical test procedures and should be used for informational purposes only.
They are not intended to represent performance specifications.
Notes on Data/Panel:
1. This panel is for Research Use Only. Not for use in diagnostic procedures.
2. Data is generated at ZeptoMetrix™ LLC and by our Laboratory Partners in the United States and Europe.
3. All specimens are unadulterated 4% sodium citrated plasma samples collected from a single donor in the United States.
4. No preservatives have been added.
5. These specimens are not a substitute for the positive and negative control reagents provided with licensed test kits.
6. The Syphilis Mixed Titer Panel is a 15 member panel consisting of vials each containing 1mL of plasma.
7. Expiration Date: 1/25/2021