Sirius Red/Fast Green Collagen Staining Kit
- SKU:
- 445-9046
- Size:
- 30-50 samples
- Shipping:
- RT
- Storage:
- RT
Description
Sirius Red/Fast Green Collagen Staining Kit - Cat Number: 9046 From Chondrex.
Research Field: Arthritis
Clonality: N/A
Cross-Reactivity:
Host Origin: N/A
Applications: N/A
Isotype: N/A
Detection Range: Collagen: 3 µg/section Other Proteins: 50 µg/section
Sample Type: N/A
Concentration: N/A
Immunogen:
PRODUCT SPECIFICATIONS
DESCRIPTION: Stain kit to quantify total collagen
FORMAT: Glass slides and tissue culture plates
ASSAY TYPE: Dye staining
ASSAY TIME: Varies depending on sample type
STANDARD RANGE: Collagen: 3 μg/section
Non-collagenous proteins: 50 μg/section
NUMBER OF SAMPLES: 30-50 samples/kit
SAMPLE TYPES: Cultured cells and tissue sections
RECOMMENDED SAMPLE DILUTIONS: N/A
CHROMOGEN: N/A (read at 540 and 605 nm)
STORAGE: Room temperature for 12 months
VALIDATION DATA: N/A
NOTES:
INTRODUCTION
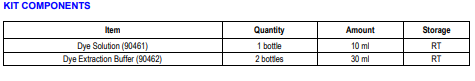
Sirius Red and Fast Green is a dye combination used to distinguish collagen from its surrounding materials. Sirius Red specifically binds the [Gly-X-Y]n helical structure of fibrillar collagens, regardless of collagen type or species, whereas Fast Green binds to non-collagenous proteins. By exploiting the unique features of these two dyes, Chondrex, Inc. provides a simple semi-quantitative assay kit to determine the amounts of collagen and non-collagenous proteins in cultured cell layers and tissue sections (1-2). Because this assay does not require collagen solubilization, it can be used to measure total collagen content in various tissues (3-7). As Sirius Red and Fast Green have absorptions at 540 nm and 605 nm respectively, the OD values of the extracted dyes can be used to calculate the collagen and non-collagenous protein content of samples. For general histological studies in which tissue sections are 10-20 μm thick, the assay sensitivity for collagen and non-collagenous proteins is greater than 3 μg/section and 50 μg/section, respectively. This kit contains enough reagents to stain 30-50 samples.
ASSAY PROTOCOL
The following sample preparation methods are standard protocols. Therefore, these protocols may need to be optimized depending on the
sample types.
A. Paraffin Embedded Tissue Sections
1. Prepare paraffin-embedded tissue sections (approximately 30-50 mm2
, 10-20 μm thick).
2. Deparaffinize the tissue sections with the following steps below:
1. Xylene, 10 minutes
2. Xylene 1:1 with 100% ethanol, 10 minutes
3. 100% ethanol, 10 minutes
4. 50% ethanol/distilled water, 5 minutes
5. Distilled water, 5 minutes
3. Transfer individual slides to petri dishes.
4. Load 0.2 - 0.3 ml Dye Solution on each sample, enough to completely immerse the tissue section, and incubate at room temperature
for 30 minutes.
NOTE: To avoid evaporation of the Dye Solution, place a piece of wet filter paper beneath the slide and cover the petri dish with a lid.
5. Carefully aspirate the Dye Solution.
6. Rinse the stained tissue section with 0.5 ml of distilled water repeatedly until the water runs clear.
OPTION: These samples may be observed under a microscope without the following extraction step. Dehydrate in 100% ethanol,
followed by a xylene wash, and mount in a resinous medium.
7. Load 1 ml of Dye Extraction Buffer on each sample and gently mix by pipetting until the color is eluted from the tissue section.
8. Collect the eluted Dye Solution and read the OD values at 540 nm and 605 nm with a spectrophotometer.
B. Frozen Tissue Sections
1. Prepare frozen tissue sections (approximately 30-50 mm2
, 10-20 μm thick) according to standard methods.
2. Wash with 1X PBS.
Optional fixing step (8,9): Add 0.5 ml of Kahle fixative, enough to completely immerse each tissue section, and incubate for 10 minutes
at room temperature.
Kahle fixative recipe
- 60 ml distilled water
- 28 ml 95-100% ethanol
- 10 ml 37% formaldehyde
- 2 ml glacial acetic acid.
3. Remove the fixative, wash with 1X PBS, and transfer individual slides to petri dishes.
4. Load 0.2 - 0.3 ml of Dye Solution on each sample, enough to completely immerse the tissue section, and incubate at room temperature
for 30 minutes.
NOTE: To avoid evaporation of the Dye Solution, place a piece of wet filter paper beneath the slide and cover the petri dish with a lid.
5. Carefully aspirate the Dye Solution.
6. Rinse the stained tissue section with 0.5 ml of distilled water repeatedly until the water runs clear.
OPTION: These samples may be observed under a microscope without the following extraction step. Dehydrate in 100% ethanol,
followed by a xylene wash, and mount in a resinous medium.
7. Load 1 ml of Dye Extraction Buffer on each sample and gently mix by pipetting until the color is eluted from the tissue section.
8. Collect the eluted Dye Solution and read the OD values at 540 nm and 605 nm with a spectrophotometer
C. In Vitro Cultured Cell Layers
1. If planning to mount cell layers and observe with a microscope before proceeding with the dye extraction step, place a sterilized round
glass slide at the bottom of each well of a 24-well culture plate. Otherwise, skip this step.
2. Culture cells in the 24-well culture plates for the required time.
3. Remove the culture media and wash the wells with 1X PBS.
4. Add 0.5 ml of Kahle fixative, enough to completely immerse the cell layers, and incubate for 10 minutes at room temperature.
Kahle fixative recipe
- 60 ml distilled water
- 28 ml 95-100% ethanol
- 10 ml 37% formaldehyde
- 2 ml glacial acetic acid
5. Remove the fixative and wash with 1X PBS.
6. Add 0.2 - 0.3 ml of Dye Solution, enough to completely immerse the fixed cell layers, and incubate at room temperature for 30 minutes.
If cells are on glass slides, remove the slides and place them in petri dishes for the staining.
NOTE: To avoid evaporation of the Dye Solution, place a piece of wet filter paper beneath the slide and cover the petri dish with a lid.
7. Carefully aspirate the Dye Solution.
8. Rinse the stained cell layers with 0.5 ml of distilled water repeatedly until the water runs clear.
OPTION: These samples may be observed under a microscope without the following extraction step. Dehydrate in 100% ethanol,
followed by a xylene wash, and mount with a resinous medium.
9. Load 1 ml of Dye Extraction Buffer on each sample and gently mix by pipetting until the color is eluted from the sample.
10. Collect the eluted dye solution and read the OD values at 540 nm and 605 nm with a spectrophotometer
1 Review
-
Finally! Affordable & Easy Collagen Staining
I highly recommend it! This stain has been a lifesaver in my lab. I've used it in many experiments, and the results are always clear. I can quickly see exactly how much collagen is in my samples, without needing any fancy equipment.













