Description
The RETRO-TEK HIV-1 ICx/CRx Kit is supplied for research purposes only. It is not intended for use in the diagnosis or prognosis of disease or for screening and may not be used as a confirmatory test in diagnostic situations.
HIV-1 p24 antigen may be detectable at the onset of an infection with the Human Immunodeficiency Virus Type 1 (HIV-1), prior to seroconversion and late in infection as the disease progresses to AIDS. However, during the ARC (AIDS Related Complex) phase of the disease, p24 antigen assays have had limited utility, since the majority of patients produce both anti-p24 antibody and p24 antigen and form immune complexes. These immune complexes may either interfere with or prevent the measurement of p24 antigen by conventional immunoassays2,3.
The RETRO-TEK HIV-1 ICx/CRx Kit complements the RETRO-TEK HIV-1 p24 Antigen ELISA (ZEPTOMETRIX Catalog #: 0801111). The ICx/CRx Kit contains two sets of reagents, each of which may provide supplemental information that enhances the detection of p24 antigen in serum or plasma:
- The Immune Complex Dissociation (ICx) Reagents may identify serum or plasma specimens in which p24 antigen is masked by antibody.
- The Confirmation Reagents (CRx) may confirm the reactivity of positive specimens.
REAGENTS
Materials Supplied:
• ICx Acid, 25 ml: Contains glycine-HCl. (Reagent A)
• ICx Base, 25 ml: Contains tris-HCl and sodium azide. (Reagent B)
• ICx Positive Control, 1 ml: Contains human source material with p24 immune complexes (heat inactivated) and sodium azide.
(Reagent C)
• ICx/CRx Negative Control, 1 ml: Contains human source material non-reactive for antibodies to HIV-1 and non-reactive for HIV-1 p24 antigen and sodium azide. (Reagent D)
• CRx Control Reagent, 1ml: Contains human source material non-reactive for antibodies to HIV-1 and non-reactive for HIV-1 p24 antigen, Triton X-100® and sodium azide. (Reagent E)
• CRx Neutralization Reagent, 1 ml: Contains human source material with antibodies to HIV-1 (heat inactivated), Triton X-100® and sodium azide. Non-reactive for HIV-1 p24 antigen. (Reagent F)
® Triton X-100 is a registered trademark of Union Carbide Chemicals and Plastics Co., Inc.
Materials Required but Not Supplied:
• Lysing Buffer, HIV-1 p24 Antigen Standard and Assay Diluent from
• RETRO-TEK HIV-1 p24 Antigen ELISA
• Disposable gloves
• Validated adjustable micropipettes, single and multichannel
• Test tubes and racks for preparing specimen and control dilutions
• Graduated cylinders and assorted beakers
• Validated automatic microplate washer or manual vacuum aspiration equipment
• Validated incubator for 37°C ±1°C
• Validated microplate reader
• Timer
• 1% sodium hypochlorite as disinfectant. May be prepared from household bleach
• Distilled or deionized water
STORAGE:
Store all kit reagents at 2° - 8°C. DO NOT FREEZE. When stored properly the kit is stable until the date indicated on the box label.
PRECAUTIONS
FOR RESEARCH USE ONLY. Not For in vitro Diagnostic Use.
• Prior to performing the assay, carefully read all instructions.
• Use universal precautions when handling kit components and test specimens.*
• To avoid cross-contamination, use separate pipet tips for each specimen.
• Do not interchange components of this kit with any other kits or reagents.
• Disposal: When testing potentially infectious human specimens, adhere to all applicable local, state and federal regulations regarding the disposal of biohazardous material.
• Human source material used in the manufacture of the HIV-1 p24 Detector
• Antibody, ICx Positive Control, ICx /CRx Negative Control, CRx Control
• Reagent and CRx Neutralization Reagent has been tested and found negative for Hepatitis B surface antigen. The viral lysate used to prepare the
• HIV-1 p24 Antigen Standard has been inactivated by chemical disruption and heating. Handle these reagents as if capable of transmitting infectious agents.
* from MMWR, June 24, 1988, Vol. 37, No. 24, pp. 377-382, 387-388.
1. IMMUNE COMPLEX DISSOCIATION
TEST PROCEDURE
Allow all reagents to reach room temperature before use. Label test tubes to be used for preparation of Positive Control, Negative Control and specimens.
Step 1: Pipet 75 µl of ICx Positive Control and 75 µl of ICx Acid into the appropriate tubes and mix. Prepare ICx/CRx Negative Control and all serum or plasma specimens in the same manner.
Step 2: Incubate for 1 hour at 37°C (±1°C).
Step 3: During incubation, prepare all reagents necessary to perform the RETRO-TEK HIV-1 p24 Antigen ELISA.
Step 4: Pipet 75 µl of ICx Base reagent into each sample or control and mix.
Step 5: Pipet 25 µl of Lysing Buffer into each test tube and mix.
Step 6: Immediately assay each specimen using the RETRO-TEK HIV-1 p24 Antigen ELISA as described on Page 4 of the p24 ELISA Product Insert. Omit Step 1 since Lysing Buffer has already been added.
IMMUNE COMPLEX DISSOCIATION PROCEDURAL FLOW CHART
ICx ACID REAGENT, CONTROLS AND SPECIMENS
=>
INCUBATE 1 HOUR AT 37°C ± 1°C
=>
PIPET ICx BASE REAGENT
=>
PIPET LYSING BUFFER
=>
PROCEED WITH RETRO-TEK HIV-1 p24 ANTIGEN ELISA
2. CONFIRMATION OF p24 ANTIGEN POSITIVE SPECIMENS BY NEUTRALIZATION
PRINCIPLE OF TEST
Samples found reactive in the RETRO-TEK HIV-1 p24 Antigen ELISA should be confirmed by neutralization. Each reactive sample is incubated separately with the CRx Neutralization and the CRx Control reagents prior to analysis in the RETRO-TEK HIV-1 p24 Antigen ELISA. During the incubation, p24 antigen present in the sample will complex with the anti-p24 antibody contained in the CRx Neutralization Reagent; conversely, no antigen-antibody complexing will occur in the sample incubated with the CRx Control Reagent. When tested in the ELISA, non-complexed antigen in the sample will be captured by the anti-p24 coated microplate; complexed antigen will not be captured by the anti-p24 coated microplate, resulting in reduction of the optical density.
In order to perform confirmation testing, samples must have a concentration of p24 antigen less than 125 pg/ml. Specimens containing more than 125 pg/ml must be diluted to a concentration of approximately 50 to 125 pg/ml prior to testing.
For confirmation of p24 antigen for specimens initially reactive without ICx treatment, proceed with Steps 1 to 4 on this page. For specimens initially reactive after ICx treatment, proceed with Steps 1 to 6 on Page 6.
TEST PROCEDURE WITHOUT ICx TREATMENT
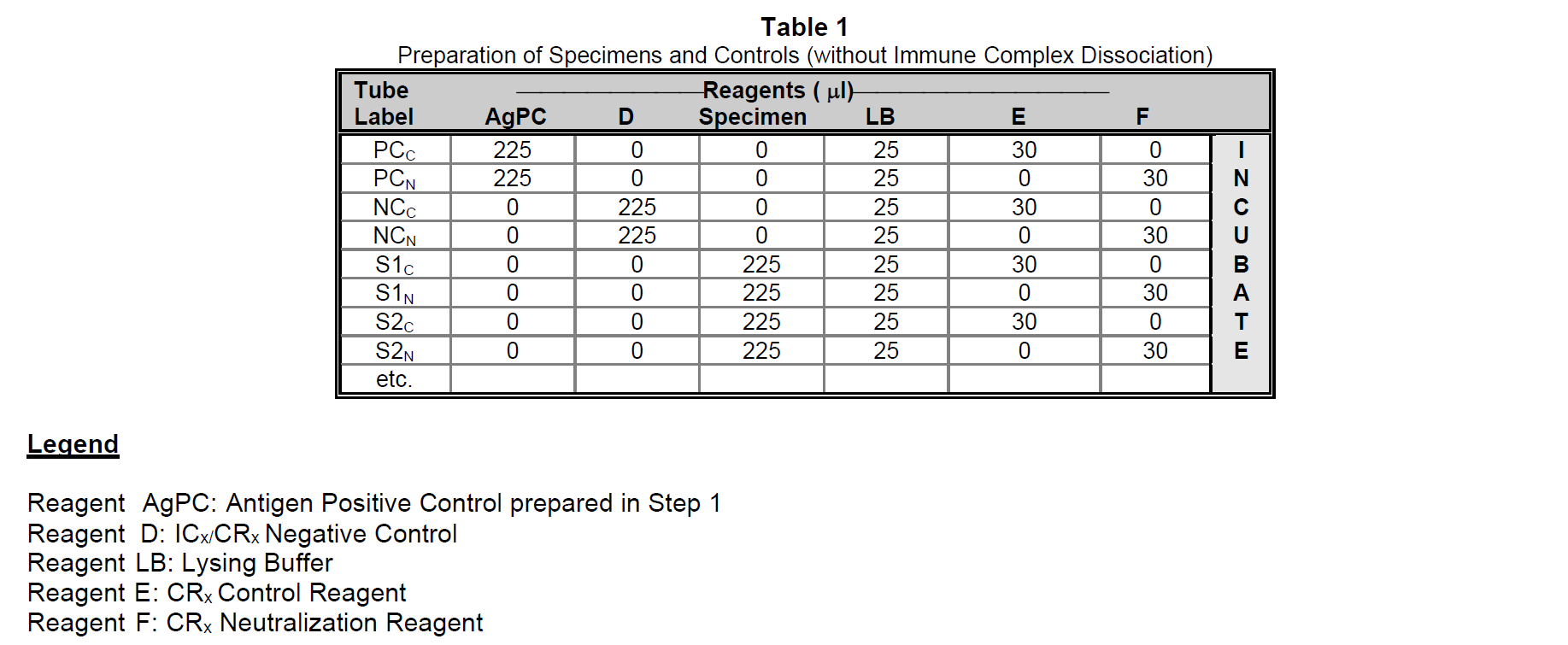
Allow all reagents to reach room temperature before use. For the following procedure, consult Table 1 for the appropriate test tube labels, reagents and volumes.
Step 1: Prepare the Antigen Positive Control (AgPC) by pipetting 25 µl of the HIV-1p24 Antigen Standard into 975 µl of Assay Diluent (62.5 pg/ml final concentration) from the RETRO-TEK HIV-1 p24 Antigen ELISA.
Step 2: Pipet 225 µl of Antigen Positive Control (AgPC), 225 µl of ICx/CRx Negative Control (Reagent D), or 225 µl of each specimen into their respective test tubes. Pipet 25 µl of Lysing Buffer from the RETRO-TEK HIV-1 p24 Antigen ELISA into each test tube. Pipet 30 µl of the CRx Control Reagent (Reagent E) or 30 µl of the CRx Neutralization Reagent (Reagent F) into their respective test tubes and mix.
Step 3: Incubate for 1 hour at 37°C (±1°C).
Step 4: Immediately assay each specimen using the RETRO-TEK HIV-1 p24 Antigen ELISA as described on Page 4 of the p24 ELISA Product Insert. Omit Step 1since Lysing Buffer has already been added.
Information regarding test validity requirements, calculations and interpretations of results is contained in the p24 ELISA Product Insert.
NEUTRALIZATION PROCEDURAL FLOW CHART WITHOUT ICX TREATMENT
PREPARE SPECIMENS, CONTROL AND REAGENTS
=>
PIPET SPECIMENS, CONTROLS AND REAGENTS
=>
INCUBATE 1 HOUR AT 37°C ± 1°C
=>
PROCEED WITH RETRO-TEK HIV-1 p24 ANTIGEN ELISA
TEST PROCEDURE WITH ICx TREATMENT
Allow all reagents to reach room temperature before use. For the following procedure, consult Table 2 for the appropriate test tube labels, reagents and volumes.
Step 1: Pipet 75 µl of ICx Positive Control (Reagent C), 75 µl of ICx/CRx Negative Control (Reagent D) or 75 µl of each specimen into their respective test tubes. Pipet 75 µl of ICx Acid (Reagent A) into their respective test tubes and mix.
Step 2: Incubate for 1 hr. at 37°C (±1°C).
Step 3: Pipet 75 µl of ICx Base (Reagent B) into each test tube and mix.
Step 4: Pipet 25 µl of Lysing Buffer (Reagent LB) from the RETRO-TEK HIV-1 p24 Antigen ELISA into each test tube and mix. Step 5: Pipet 10 µl of the CRx Control Reagent (Reagent E) or 10 µl of the CRx Neutralization Reagent (Reagent F) into their espective tubes and mix.
Step 6: Incubate for 1 hr. at 37ºC (±1°).Step 7: Immediately assay each specimen using the RETRO-TEK HIV-1 p24 Antigen ELISA as described on Page 4 of the p24 ELISA Product Insert. Omit Step 1since Lysing Buffer has already been added.
Information regarding test validity requirements, calculations and interpretations of results is contained in the p24 ELISA Product Insert.

NEUTRALIZATION PROCEDURAL FLOW CHART WITH ICX TREATMENT
PREPARE SPECIMENS, CONTROL, AND REAGENTS
=>
PIPET SPECIMENS, CONTROLS, AND REAGENT A
=>
INCUBATE 1 HOUR AT 37C 1C
=>
PROCEED WITH RETRO-TEK HIV-1 P24 ANTIGEN ELISA









