Description
The RETRO-TEK HIV-1 p24 Extended Range Kit is supplied for research purposes only. It is not intended for use in the diagnosis or prognosis of disease or for screening and may not be used as a confirmatory test in diagnostic situations. It is to be used as an accessory to the HIV-1 p24 ELISA or HIV-1 p24 ELISA 2.0 kit.
Zeptometrix | 0801137 | HIV-1 p24 Extended Range Kit DataSheet
PRINCIPLE OF THE TEST
Many specimens and tissue culture samples contain HIV-1 viral loads greater than the range of the standard curve of commercially available antigen ELISA's. In order to quantify such samples, a series of expensive and time consuming dilutions must be made to adjust the sample concentration. The RETRO-TEK HIV-1 p24 Extended Range Kit includes reagents that extend the standard curve of the up to 4 ng/mL for either the HIV-1 p24 ELISA (catalog #: 0801111 or 0801200) or the HIV-1 p24 Antigen ELISA 2.0 (catalog #: 0801002 or 0801008). Therefore, samples containing p24 antigen up to 4 ng/mL may be accurately quantified without extensive serial dilution.
REAGENTS
Materials Supplied
• Concentrated HIV-1 p24 Antigen Standard (0.3mL): Contains detergent disrupted, heat inactivated viral antigen at a concentration of 80 ng/mL, added protein, detergent and 2-chloroacetamide.
• Substrate (5 sets): SIGMAFASTTM OPD tablet sets.
Contains: 5 OPD tablets (o-phenylenediamine dihydrochloride) [silver foil]
Contains: 5 urea-hydrogen peroxide tablets [gold foil]
SIGMAFASTTM is a registered trademark of the Sigma-Aldrich Company.
Storage: Store all kit reagents at 2°-8°C. Materials Required but Not Supplied
• HIV-1 p24 Antigen ELISA (catalog #: 0801111 or 0801200) or
HIV-1 p24 Antigen ELISA 2.0 (catalog #: 0801002 or 0801008)
• Distilled or deionized water
• Test tubes and racks for preparing specimen and standard dilutions
• Adjustable micropipettes, single and multichannel
• Incubator capable of maintaining 37º± 1º C
• Timer
• Graduated cylinders and assorted beakers
• Automatic microplate washer or manual vacuum aspiration equipment
• 1% sodium hypochlorite as disinfectant. May be prepared from household bleach
PRECAUTIONS
• OPD is a possible carcinogen. Avoid contact. Use gloves when handling tablets.
• Use Universal Precautions when handling test specimens and HIV-1 p24 Antigen Standard.*
• Disposal: When examining human source material or other potentially infectious specimens, adhere to all applicable local, state and federal regulations regarding disposal of hazardous materials.
• To avoid cross-contamination, use separate pipet tips for each specimen.
* from MMWR, June 24,1988, Vol. 37, No.24, pp. 377-382, 387-8
PREPARATION OF REAGENTS
Extended Range Standard Curve: Prepare a series of seven standards from the Concentrated HIV-1 p24 Antigen Standard as shown in Table 1.
Any diluted standards remaining after the completion of the assay should be discarded.
Do not save diluted reagent.
OPD Substrate Solution: Dissolve 1 OPD tablet and 1 urea hydrogen peroxide tablet in 11.0 mL of distilled or deionized water. Prepare at least 5 minutes in advance to using as it will take this long to dissolve both tablets. Protect from light until use. Use the solution within 15 minutes of its preparation. Do not save diluted reagent.
TEST PROCEDURE
Allow all reagents to reach room temperature before use. Proceed with the RETRO-TEK HIV-1 p24 ELISA according to the product insert with the following changes:
1. Replace the HIV-1 p24 Antigen Standard supplied with the p24 ELISA kit with the HIV-1 p24 Antigen Extended Range Standard.
2. Substitute the TMB Substrate supplied with the p24 ELISA kit with the OPD Substrate Solution and incubate microplate for 30 minutes at room temperature.
Please Note: Due to plate reader differences, the high standard absorbance values may be out of range. Read plate at 435nm or 405nm if necessary.
CALCULATION AND INTERPRETATION OF RESULTS
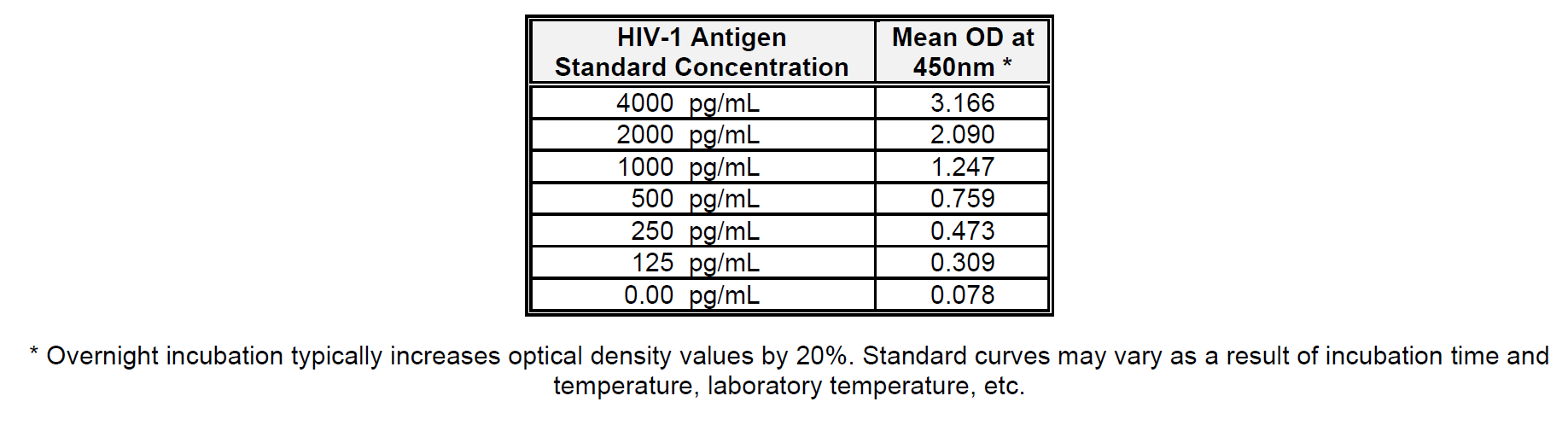
Calculate the mean absorbance for each p24 standard and test sample. Plot the concentration of HIV-1 p24 Antigen Standard (pg/mL) on the X-axis versus the mean optical density (OD) for each standard on the Y-axis. The HIV-1 p24 concentration of unknown samples is best determined by using a 4-parameter logistic regression. Alternatively, a point-to-point algorithm may be used. Only readings which fall within the range of the standard curve may be quantified. Be sure to correct for all dilutions, including the 1.1 dilution made during the addition of Lysing Buffer.
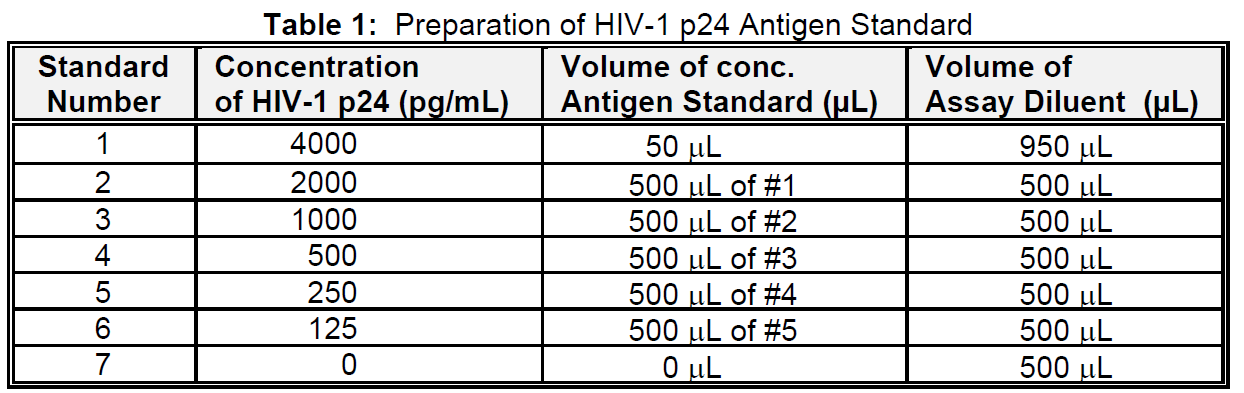
Below is an example of a typical standard curve for the RETRO-TEK HIV-1 p24 Antigen ELISA (ZEPTOMETRIX catalog # 0801111 or 0801200).
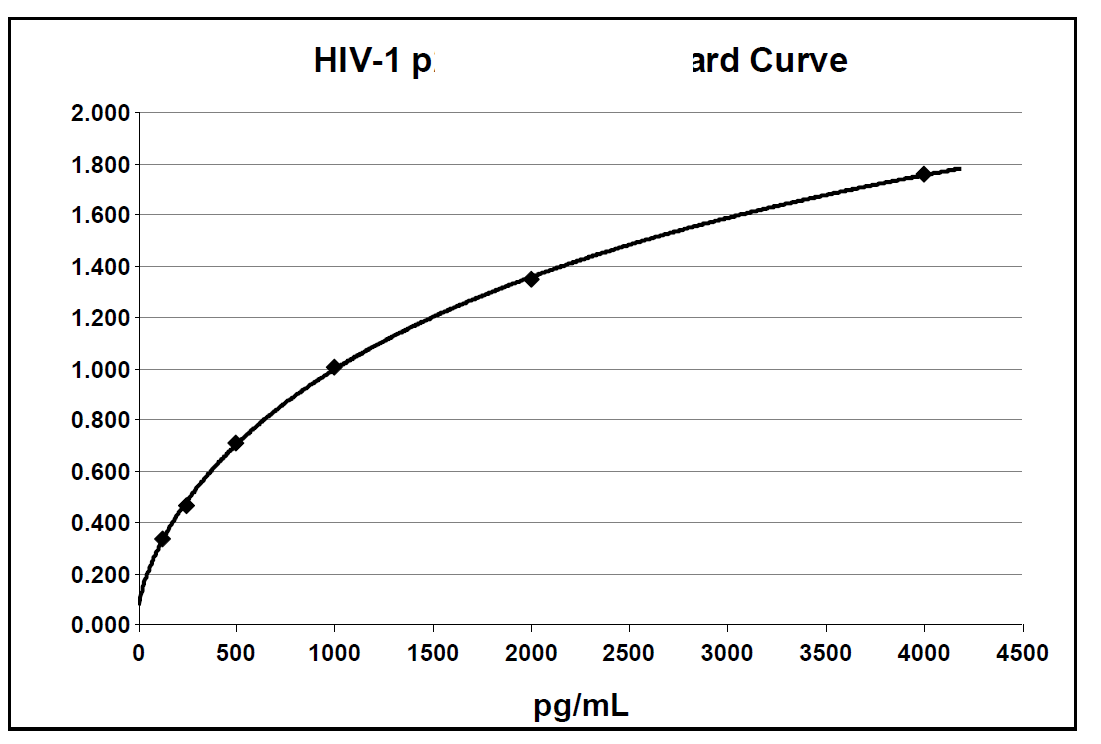
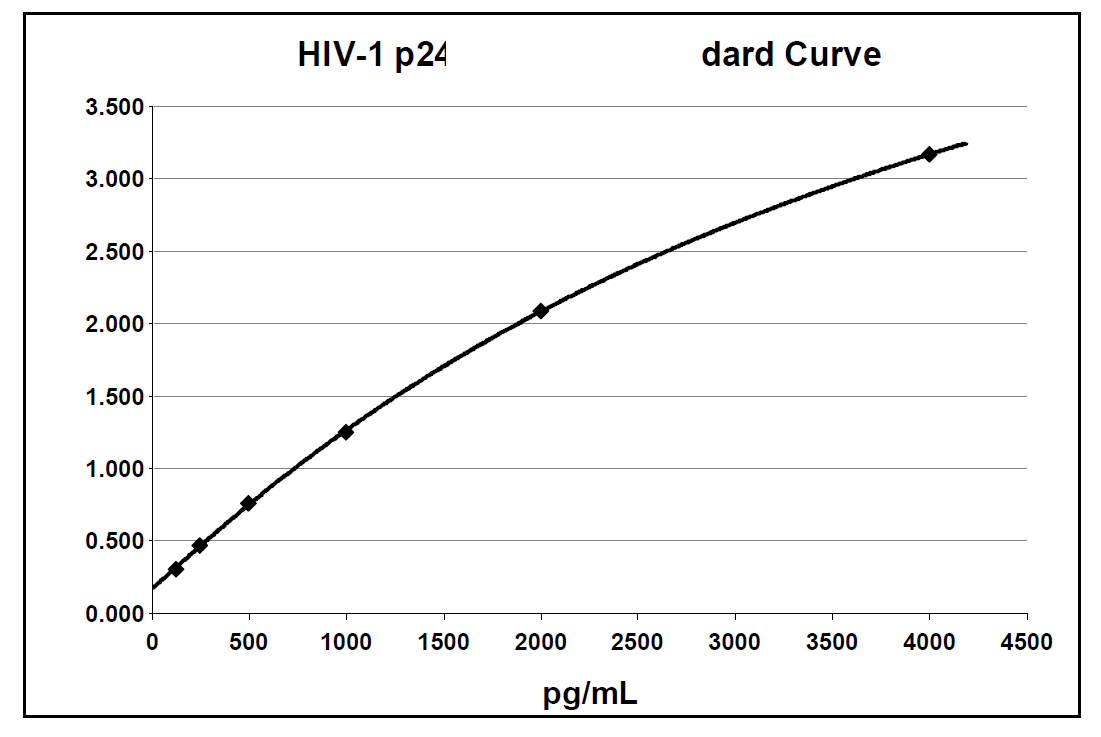
Below is an example of a typical standard curve for the RETRO-TEK HIV-1 p24 Antigen ELISA 2.0 (ZEPTOMETRIX catalog # 0801002 or 0801008).
TEST VALIDITY
• The optical density of the 0.0 pg/mL standard should not be above 0.120 otherwise the test is invalid.
• The optical density values of the 4000 pg/mL p24 standard should be greater than 1.000 otherwise the test is invalid
LIMITATIONS OF THE PROCEDURE
Serum or plasma specimens from most HIV-1 infected individuals contain antibodies to the p24 antigen. The concentration and microspecificity of these antibodies will vary from individual to individual and from bleed to bleed. The observed level of p24 antigen in any specimen containing p24 antibodies may be affected by the following:
• Host antibodies may mask the epitope reactive with capture monoclonal antibody. Consequently, the optical density may be reduced.
• Host antibodies may mask epitopes that bind to the detector antibody, again reducing the optical density.
• After immune complex dissociation and neutralization, host antibodies may recombine with p24 antigen. The recombination of host antibody with antigen is competitive with the capture of p24 by the monoclonal antibody on the solid phase and thus may again mask some of the p24. The rate of recombination is determined by the concentrations of p24 antigen and anti-p24 antibodies, the affinity of the antibodies, as well as temperature and time and is therefore difficult to control. Correlation of results for specimens between repeat test runs and among kits from different manufacturers may be adversely affected by such competitive recombination.
PROCEDURAL FLOW CHART
PREPARE REAGENTS
=>
WASH PLATE
=>
PIPETTE SPECIMENS, STANDARDS
AND CONTROLS
=>
FOLLOW HIV-1 p24 ELISA PROCEDURE
=>
PIPETTE OPD SUBSTRATE SOLUTION
=>
INCUBATE 30 MINUTES AT ROOM TEMPERATURE
=>
PIPETTE STOP SOLUTION
=>
READ AT 450NM
FOR RESEARCH USE ONLY.
NOT FOR USE IN DIAGNOSTIC PROCEDURES.










