Description
The RETRO-TEK HTLV p19 Antigen ELISA is supplied for research purposes only. It is not intended for use in the diagnosis or prognosis of disease, or for screening, and may not be used as a confirmatory test in diagnostic situations.
The RETRO-TEK HTLV p19 Antigen ELISA is an enzyme linked immunoassay for the detection of Human T-Lymphotropic Virus Type I (HTLV-I) and Type II (HTLV-II) core antigen in test specimens. The assay is useful in monitoring the course of in vitro expression of HTLV-I and HTLV-II in cell cultures and to monitor the purification and biochemical behavior of HTLV-I and HTLV-II.
The RETRO-TEK HTLV p19 Antigen ELISA may augment and/or supplant reverse transcriptase (RT) measurements traditionally used to assess the presence of retroviruses. Such enzymatic measurements, however, are not HTLV-I or HTLV-II specific. In contrast, the RETRO-TEK HTLV p19 Antigen ELISA is immunologically specific for HTLV-I and HTLV-II, uses no radioactive components and is more sensitive than RT.
Zeptometrix | 0801116 | HTLV p19 Antigen ELISA Datasheet
PRINCIPLE OF THE TEST
Microwells coated with high affinity polyclonal antibodies form the capture phase of the assay. These antibodies react strongly with the major gag gene products of HTLV-I and HTLV-II. Viral antigen in the test specimen is captured by the antibody during the sample incubation step. Captured antigen reacts with Detector Antibody which recognizes p19 core protein of HTLV-I and HTLV-II. Specifically, bound Detector Antibody is detected with peroxidase conjugated lgG and color is developed with 3,31,5,51, tetra-methylbenzidine (TMB) as substrate. Resultant absorbance values are proportional to the amount of viral core antigen present in the test specimens.
REAGENTS
Materials Supplied:
• HTLV Antibody Coated Microplate (1 plate): 96 well microplate coated with polyclonal antibodies.
• HTLV Detector Antibody (0.5ml): Antibodies to HTLV-I and HTLV-II p19 core proteins. Contains added protein, Triton X-100® and 2-chloroacetamide.
• HTLV Antigen Standard (0.5ml): Detergent-disrupted, heat-inactivated viral antigen at a concentration of 16 ng/ml p19. Contains added protein, Triton X-100® and sodium azide.
• Lysing Buffer (5ml): Triton X-100® in PBS and 2-chloroacetamide.
• Peroxidase Reagent (0.3ml): Peroxidase conjugated lgG. Contains added protein, Triton X-100® and 2-chloroacetamide.
• Assay Diluent (100ml): Contains PBS, added protein, Triton X-100® and 2-chloroacetamide.
• 10X Plate Wash Buffer (125ml): Contains PBS, Tween 20® and 2-chloroacetamide.
• Substrate (0.3 ml): Tetramethylbenzide (TMB) solution in dimethyl sulfoxide.
• Substrate Buffer (50ml): Contains citrate/acetate buffer, hydrogen peroxide and 2- chloroacetamide.
• Stop Solution (12ml): Proprietary formulation
• Resealable Plastic Bag: 1
® Triton X-100 is a registered trademark of Union Carbide Chemicals and Plastics Co., Inc. Tween 20 is a registered trademark of Imperial Chemical Industries.
Materials required but not supplied:
• Test tubes and racks for preparing specimen and control dilutions
• Validated adjustable micropipettes, single and multichannel
• Distilled or deionized water
• Validated incubator capable of maintaining 37º ± 1ºC
• Timer
• Graduated cylinders and assorted beakers
• Validated microplate reader
• Automatic microplate washer or manual vacuum aspiration equipment
• 1% sodium hypochlorite as disinfectant. May be prepared from household bleach
STORAGE
Store all kit reagents at 2° - 8°C. DO NOT FREEZE. When stored properly the kit is stable until the date indicated on the box label.
PRECAUTIONS
FOR RESEARCH USE ONLY. Not for in vitro Diagnostic Use.
• Use Universal Precautions* when handling test specimens and when performing this test *(from MMWR, June 24,1988, Vol. 37, No.24, pp. 377-382, 387-388)
• When examining human source material or other potentially infectious specimens, adhere to all applicable local, state and federal regulations regarding disposal of hazardous materials. To avoid cross-contamination, use separate pipet tips for each specimen.
• The HTLV Antigen Standard contains sodium azide as a preservative. Sodium azide may react with lead or copper pipes to form explosive metal azides. Flush pipes with large quantities of water upon disposal to prevent azide buildup in drains.
• Stop Solution contains hydrochloric acid which may cause severe burns. In case of contact with eyes or skin, rinse immediately with water and seek medical assistance. Wear protective clothing and eyewear.
PREPARATION OF REAGENTS
HTLV ANTIGEN STANDARD: Prepare a series of six standards, in Assay Diluent, from the HTLV Antigen Standard. The dilution scheme in Table 1 is recommended.
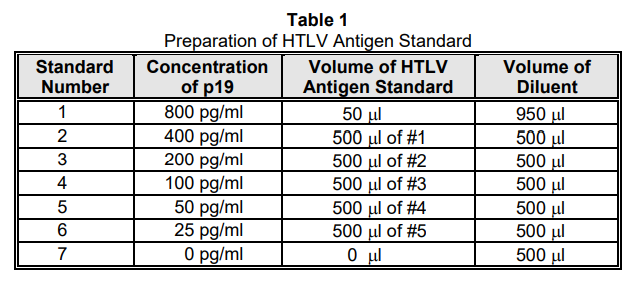
Any diluted HTLV Antigen Standard remaining after the completion of the assay should be discarded. Do not save diluted reagent.
HTLV DETECTOR ANTIBODY, PEROXIDASE, AND SUBSTRATE working solutions: 1/100 final dilution. The dilution scheme in Table 2 is recommended. Dilute HTLV Detector Antibody and Peroxidase Reagent in Assay Diluent. Dilute Substrate in Substrate Buffer. Do not save diluted reagents.
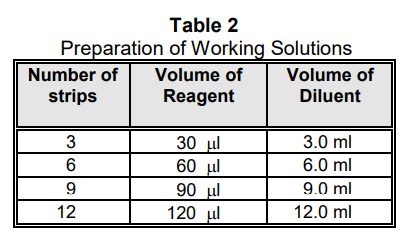
PLATE WASH BUFFER: Dilute 1:10 in distilled or deionized water prior to use. Mix thoroughly. Prepared 1X Plate Wash Buffer may be stored at 2º-8°C for up to 1 week. Additional Wash Buffer (ZMC Catalog No. 0801060) may be ordered.
TEST PROCEDURE
Allow all reagents to reach room temperature before use. If the entire 96 well plate is not used, remove surplus strips from the plate frame. Place surplus strips and desiccant into the Resealable Plastic Bag, and store at 2º-8ºC.
Label each strip on its end tab to ensure identity should the strips become detached from the plate frame during the assay.
Step 1: Specimens to be tested (serum, culture fluids, chromatographic or ultracentri-fugation fractions, etc.) must be treated with Lysing Buffer. Pipet 50 µl of Lysing Buffer into 450 µl of specimen. Mix well.
Step2: Wash the microplate prior to the addition of samples. Fill each well with 300 µl of 1X Wash Buffer and aspirate. Perform 6 fill/aspirate cycles. After final wash cycle, thoroughly blot by carefully striking the inverted microplate on a pad of absorbent towels placed on the bench top. Continue striking until no droplets remain in the wells. Do not allow washed plates to completely dry prior to sample addition. Drying will adversely affect test results.
Step 3: Designate one well of the microplate, and leave empty. This well is used for background determination (substrate blank).
Step 4: Pipet 200 µl of standards #1-7 into duplicate wells.
Step 5: Pipet 200 µl of each specimen into separate duplicate wells.
Step 6: Cover the microplate with a sealer and incubate the plate for 2 hours at 37ºC.
Step 7: Aspirate the contents of each well; wash plate as described in Step 2.
Step 8: Pipet 100 µl of HTLV Detector Antibody working solution to each well of the microplate except the substrate blank which is left blank. See Preparation of Reagents for dilution information. Cover and incubate for one hour at 37C.
Step 9: Aspirate the contents of each well; wash plate as described in Step 2.
Step 10: Pipet 100 µl of the Peroxidase working solution into each well except the substrate blank which is left blank.
See Preparation of Reagents for dilution information. Cover and incubate for 1 hour at 37°C.
Step 11: Aspirate the contents of each well; wash plate as described in Step 3.
Step 12: Pipet 100 µl of Substrate Solution into each well and incubate uncovered for thirty minutes at room temperature (18-25°C). A blue color will develop in wells containing viral antigen.
Step 13: Stop the reaction by pipetting 100 µl Stop Solution into each well. A color change from blue to yellow will result.
Step 14: Within fifteen minutes, read the optical density of each well at 450 nm using a microplate reader.
TEST VALIDITY
Determine the mean optical density values for each standard and specimen. For the test to be valid, it must meet the following criteria:
• The mean optical density of the 0 pg/ml standard and the substrate blank must be less than 0.200.
• The mean optical density of the 200 pg/ml standard must be greater than or equal to 0.500
CALCULATION OF RESULTS
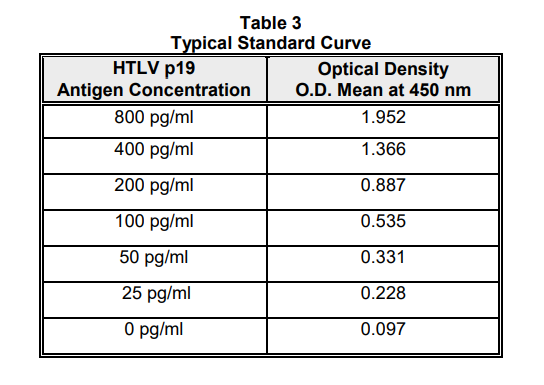
Using linear graph paper, plot mean optical densities for each standard used on the Y axis versus the corresponding concentration of HTLV p19 (pg/ml) on the X axis.
Determine the concentration of HTLV p19 in specimens by interpolation from the standard curve. Correct sample values for the 1.1-fold dilution made by the addition of Lysing Buffer and for any other dilutions performed during specimen preparation.
TYPICAL STANDARD CURVE: This is an example of a typical standard curve. Variation may occur in individual labs due to pipetting, laboratory and incubator temperatures, etc.
PROCEDURAL FLOW CHART
PREPARE REAGENTS
=>
WASH PLATE
=>
PIPET SPECIMENS AND CONTROLS
=>
INCUBATE AT 37ºC FOR 2 HOURS
=>
WASH PLATE
=>
PIPET DETECTOR ANTIBODY
=>
INCUBATE AT 37ºC FOR 1 HOUR
=>
WASH PLATE
=>
PIPET PEROXIDASE
WORKING SOLUTION
=>
INCUBATE AT 37°C FOR 1 HOUR
=>
WASH PLATE
=>
PIPET SUBSTRATE SOLUTION
=>
INCUBATE AT ROOM TEMPERATURE FOR 30 MINUTES
=>
STOP AND READ PLATE
3 Reviews
-
imballaggio superbo
quando ho ricevuto il mio pacco e l'ho aperto, sono stato molto contento che abbiano imballato i loro prodotti in modo così esperto che i prodotti rimarranno al sicuro da eventuali danni. Sono davvero contento della loro superba confezione
-
Envío rápido
Me gustaría agradecer a Genatur por su envío súper rápido y rápido. Debo decir que el personal de Genatur está bien capacitado y es responsable.
-
good support
The compay support to its users is very good, gives quick response to customers and solve the queries of its buyres and customers.






