Description
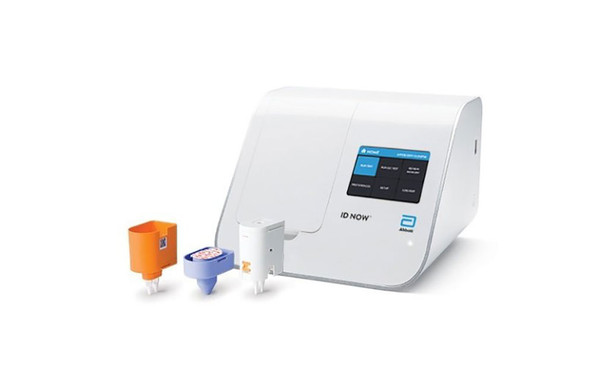
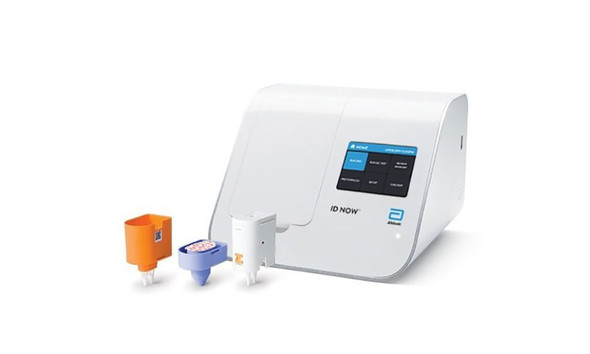
Abbott™ ID NOW RSV Test Kit Highly sensitive rapid diagnostic tests for RSV are needed to help guide management in the clinical setting. Combines the benefits of speed and accuracy on an easy to use system.
Description
- Rapid molecular in vitro diagnostic test utilizing isothermal Nucleic Acid Amplification Technology for the qualitative detection of Respiratory Syncytial Virus l nucleic acid in nasopharyngeal swabs or nasopharyngeal swabs
- Eluted in Viral Transport Media (VTM)
- Intended to aid in the rapid diagnosis of Respiratory Syncytial Virus (RSV)
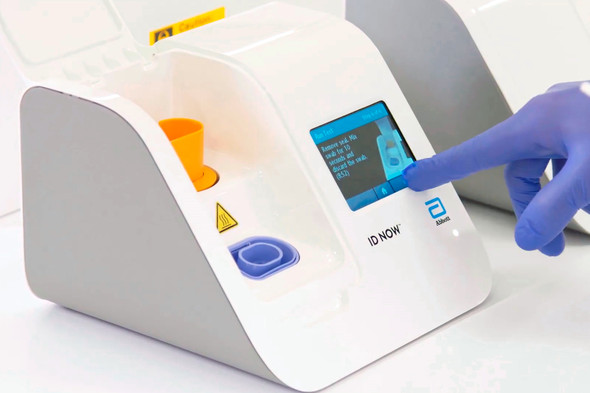
- Delivers molecular RSV results in 13 minutes or less on the unique Abbott™ ID NOW platform.
- Allows to make real-time clinical decisions which impact patient care
Certifications
CLIA-waived
Specifications
| 13 min. or less | |
| CLIA waived | |
| Direct NPS: 98.6%/NPS eluted in VTM: 98.6% | |
| Nasopharyngeal Swab, Nasopharyngeal swab eluted in Viral Transport Media (VTM) | |
| 24/Pk. | |
| 4 Months at launch, will increase | |
| Yes | |
| Already Listed | |
| Test Kit | |
| Length - 11.125 in., Width - 8.5 in., Height - 8.3125 in. |
| Abbott ID NOW | |
| Direct NPS: 98%/NPS eluted in VTM: 97.8% | |
| For diagnosis of Respiratory Syncytial Virus (RSV) | |
| Cardboard | |
| Room Temperature | |
| CLIA waived | |
| 87801 | |
| Cardboard Box | |
| 24/Pk. | |
| Stable: Box 1 - powder Box 2 - Liquid |
1 Review
-
perfetta
Ho ordinato un antibiotico per mia nonna e il risultato di quel prodotto è stato eccezionale. Davvero impressionato