Description
Hepatitis B Surface Antigen Rapid Test Device (Whole Blood/Serum/Plasma) | ABON1000646 | Abon Biopharm
Intended Use
Rapid Test Device (Whole Blood/Serum/Plasma) is a rapid chromatographic immunoassay for the qualitative detection of Hepatitis B Surface Antigen in whole blood, serum or plasma.
SUMMARY
Viral hepatitis is a systemic disease primarily involving the liver. Most cases of acute viral hepatitis are caused by Hepatitis A virus, Hepatitis B virus (HBV) or Hepatitis C virus. The complex antigen found on the surface of HBV is called HBsAg. Previous designations included the Australia or Au antigen.1 The presence of HBsAg in whole blood, serum or plasma is an indication of an active Hepatitis B infection, either acute or chronic. In a typical Hepatitis B infection, HBsAg will be detected 2 to 4 weeks before the ALT level becomes abnormal and 3 to 5 weeks before symptoms or jaundice develop. HBsAg has four principal subtypes: adw, ayw, adr and ayr. Because of antigenic heterogeneity of the determinant, there are 10 major serotypes of Hepatitis B virus.2 The HBsAg Hepatitis B Surface Antigen Rapid Test Device (Whole Blood/Serum/Plasma) is a rapid test to qualitatively detect the presence of HBsAg in whole blood, serum or plasma specimen. The test utilizes a combination of monoclonal and polyclonal antibodies to selectively detect elevated levels of HBsAg in whole blood, serum or plasma.
PRINCIPLE
The HBsAg Hepatitis B Surface Antigen Rapid Test Device (Whole Blood/Serum/Plasma) is a qualitative, solid phase, two-site sandwich immunoassay for the detection of HBsAg in whole blood, serum or plasma. The membrane is pre-coated with anti-HBsAg antibodies on the test line region of the Device. During testing, the whole blood, serum or plasma specimen reacts with anti-HBsAg antibodies conjugated particles. The mixture migrates upward on the membrane chromatographically by capillary action to react with anti-HBsAg antibodies on the membrane and generate a colored line. The presence of this colored line
in the test region indicates a positive result, while its absence indicates a negative result. To serve as a procedural control, a colored line will always appear in the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
REAGENTS
The test device contains anti-HBsAg particles and anti-HBsAg coated on the membrane.
PRECAUTIONS
• For professional in vitro diagnostic use only. Do not use after expiration date.
• Do not eat, drink or smoke in the area where the specimens or kits are handled.
• Do not use test if pouch is damaged.
• Handle and dispose all specimens and materials used to perform the test as if they contained
infectious agents. Observe established precautions against microbiological hazards throughout all the procedures and follow the standard procedures for proper disposal of specimens.
• Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are being tested.
• The used test should be discarded according to local regulations.
• Humidity and temperature can adversely affect results.
• EDTA-K2/Sodium citrate/Potassium oxalate/Sodium heparin can work with the product after anticoagulant study and are hence recommended to use when necessary.
STORAGE AND STABILITY
The kit can be stored at room temperature or refrigerated (2-30°C). The test device is stable through the expiration date printed on the sealed pouch. The test device must remain in the sealed pouch until use. DO NOT FREEZE. Do not use beyond the expiration date.
SPECIMEN COLLECTION AND PREPARATION
• The HBsAg Hepatitis B Surface Antigen Rapid Test Device (Whole Blood/Serum/Plasma) can be performed using whole blood (from venipuncture or fingerstick), serum, or plasma.
• To collect Fingerstick Whole Blood specimens:
• Wash the patient’s hand with soap and warm water or clean with an alcohol swab. Allow to dry.
• Massage the hand without touching the puncture site by rubbing down the hand towards the fingertip of the middle or ring finger.
• Puncture the skin with a sterile lancet. Wipe away the first sign of blood.
• Gently rub the hand from wrist to palm to finger to form a rounded drop of blood over the puncture site.
• Add the Fingersitck Whole Blood specimen to the test device by using a capillary tube:
• Touch the end of the capillary tube to the blood until filled to approximately 75 μL. Avoid air bubbles.
• Place the bulb onto the top end of the capillary tube, then squeeze the bulb to dispense the
whole blood to the specimen well (S) of the test device.
• Add the Fingersitck Whole Blood specimen to the test device by using hanging drop:
• Position the patient’s finger so that the drop of blood is just above the specimen well (S) of the test
device.
• Allow 3 hanging drops of fingerstick whole blood to fall into the center of specimen well (S) on the test device, or move the patient’s finger so that the hanging drop touches the center of
the specimen well (S). Avoid touching the finger directly to the specimen well (S).
• Separate serum or plasma from blood as soon as possible to avoid hemolysis. Use only clear, nonhemolyzed specimens.
• Testing should be performed immediately after specimen collection. Do not leave the specimens at room temperature for prolonged periods. Serum and plasma specimens may be stored at 2-8°C for up to 3 days. For long term storage, specimens should be kept below -20°C. Whole blood collected by venipuncture should be stored at 2-8°C if the test is to be run within 2 days of collection. Do not freeze whole blood specimens. Whole blood collected by fingerstick should be tested immediately.
• Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. Specimens should not be frozen and thawed repeatedly.
• If specimens are to be shipped, they should be packed in compliance with federal regulations covering the transportation of etiologic agents.
MATERIALS
Materials Provided
•Test devices
• Disposable specimen droppers
• Package insert
• Buffer (for whole blood only)
Materials Required But Not Provided
• Specimen collection containers
• Lancets (for fingerstick whole blood only)
• Centrifuge
• Timer
• Disposable heparinized capillary tubes and dispensing bulb (for fingerstick whole blood only).
DIRECTIONS FOR USE
Allow the test device, specimen, buffer, and/or controls to equilibrate to room temperature (15-30°C) prior to testing.
1. Remove the test device from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed immediately after opening the foil pouch.
2. For Serum or Plasma specimen: Hold the dropper vertically, transfer 3 drops of serum or plasma (approximately 75 μL) to the specimen well (S) of test device and then start the timer. See illustration below. For Venipuncture Whole Blood specimen:
Hold the dropper vertically, transfer 3 drops of venipuncture whole blood (approximately 75 μL) to the specimen well (S) of test device, then add 1 drop of buffer (approximately 40 μL) and start the timer. See illustration below.
For Fingerstick Whole Blood specimen:
• To use a capillary tube: Fill the capillary tube, transfer approximately 75 μL of fingerstick whole blood specimen to the specimen well (S) of test device, then add 1 drop of buffer (approximately 40 μL) and start the timer. See illustration below.
• To use hanging drop: Allow 3 hanging drops of fingerstick whole blood specimen (approximately 75 μL) to the specimen well (S) of test device, then add 1 drop of buffer (approximately 40 μL) and start the timer. See illustration below.
3. Wait for the colored line(s) to appear. The result should be read at 15 minutes. Do not interpret the result after 30 minutes.
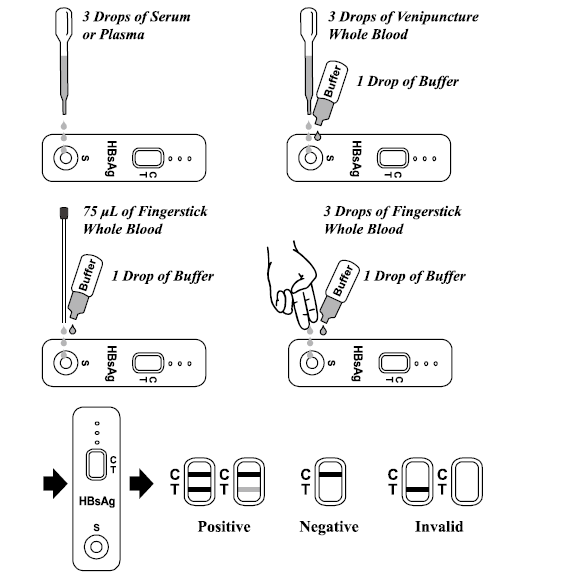
INTERPRETATION OF RESULTS (Please refer to the illustration above)
POSITIVE:* Two distinct colored lines appear. One line should be in the control region (C) and another line should be in the test region (T).
*NOTE: The intensity of the color in the test line region (T) will vary depending on the concentration of HBsAg present in the specimen. Therefore, any shade of color in the test region (T) should be considered positive.
NEGATIVE: One colored line appears in the control region (C). No apparent colored line appears
in the test region (T).
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test Device. If the problem persists, discontinue using the test kit immediately and contact
your local distributor.
the test. A colored line appearing in the control region (C) is the
internal procedural control. It confirms sufficient specimen volume and correct procedural technique.
Control standards are not supplied with this kit; however, it is recommended that a positive control
(containing 10 ng/mL HBsAg) and a negative control (containing 0 ng/mL HBsAg) be tested as a
good laboratory practice to confirm the test procedure and to verify proper test performance.
LIMITATION
1. The HBsAg Hepatitis B Surface Antigen Rapid Test Device (Whole Blood/Serum/Plasma) is for in vitro diagnostic use only. The test should be used for the detection of HBsAg in whole blood, serum or plasma specimen. Neither the quantitative value nor the rate of HBsAg concentration can be determined by this qualitative test.
2. The HBsAg Hepatitis B Surface Antigen Rapid Test Device (Whole Blood/Serum/Plasma) will only indicate the presence of HBsAg in the specimen and should not be used as the sole criteria for the diagnosis of Hepatitis B viral infection.
3. As with all diagnostic tests, all results must be considered with other clinical information available to the physician.
4. The HBsAg Hepatitis B Surface Antigen Rapid Test Device (Whole Blood/Serum/Plasma) cannot detect less than 1 ng/mL of HBsAg in specimens. If the test result is negative and clinical symptoms persist, additional follow-up testing using other clinical methods is suggested. A negative result at any time does not preclude the possibility of Hepatitis B infection.
not preclude the possibility of Hepatitis B infection.
EXPECTED VALUES
The HBsAg Hepatitis B Surface Antigen Rapid Test Device (Whole Blood/Serum/Plasma) has been compared with a leading commercial HBsAg EIA test. The correlation between these two systems is 99.4%.
PERFORMANCE CHARACTERISTICS
Sensitivity
The HBsAg Hepatitis B Surface Antigen Rapid Test Device (Whole Blood/Serum/Plasma) was tested against a sensitivity panel including both ad and ay subtypes with concentrations ranging from 0 to 300 ng/mL. The test can detect 1 ng/mL of HBsAg in whole blood, serum or plasma in 15 minutes.
Specificity
Antibodies used for the HBsAg Hepatitis B Surface Antigen Rapid Test Device (Whole Blood/Serum/Plasma) were developed against whole Hepatitis B antigen isolated from Hepatitis B virus. Specificity of the HBsAg Hepatitis B Surface Antigen Rapid Test Device (Whole Blood/Serum/Plasma) was also tested with laboratory strains of Hepatitis A and Hepatitis C. They all yielded negative results.

Precision
Intra-Assay
Within-run precision has been determined by using 10 replicates of eight specimens containing 0 ng/mL, 2 ng/mL, 5 ng/mL and 10 ng/mL both of Ad and Ay of HBsAg. The negative and positive values were correctly identified >99% of the time.
Inter-Assay Between-run precision has been determined by using the same eight specimens of 0 ng/mL, 2 ng/mL, 5 ng/mL and 10 ng/mL both of Ad and Ay of HBsAg in 3 independent assays. Three different lots of the HBsAg Hepatitis B Surface Antigen Rapid Test Device (Whole Blood/Serum/Plasma) have been tested using negative, low positive and high positive specimens. The specimens were correctly identified >99% of the time.








