Aryl Hydrocarbon Receptor (AhR) | IB06001
- SKU:
- IB06001-32 | IB06001 | IB06002
- Availability:
- In Stock
- Storage:
- -80°C
- Shipping:
- -80°C (International: -80°C)
Description
Aryl Hydrocarbon Receptor (AhR) | IB06001
This Human Aryl Hydrocarbon Receptor (AhR) assay kit is an all-inclusive firefly luciferase reporter assay system that includes in addition to AhR Reporter Cells, two optimized media for use during cell culture and in diluting the user’s test samples, a reference agonist (MeBIO), Luciferase Detection Reagent, and a cell culture-ready assay plate.
AhR Reporter Cells are prepared using INDIGO’s proprietary CryoMite™ process. This cryo-preservation method yields exceptional cell viability post-thaw, and provides the convenience of immediately dispensing healthy, division-competent reporter cells into assay plates. There is no need for cumbersome intermediate treatment steps such as spin-and-rinse of cells, viability determinations, cell titer adjustments, or the pre-incubation of reporter cells prior to assay setup.
INDIGO’s AhR assay kits feature a luciferase detection reagent specially formulated to provide stable light emission between 5 and 90+ minutes after initiating the luciferase reaction. Incorporating a 5-minute reaction-rest period ensures that light emission profiles attain maximal stability, thereby allowing assay plates to be processed in batch. By doing so, the signal output from all sample wells, from one plate to the next, may be directly compared within an experimental set.
AhR assay kits are offered in different assay formats to accommodate researchers' needs: 3x 32 and 1x 96 assay formats for screening small numbers of test compounds, as well as custom bulk reagents for HTS applications.
Assay Kit & Platforms
Bulk assay reagents can be custom manufactured to accommodate any scale of HTS. Please inquire.
Assay Services
The primary application of INDIGO’s cell-based nuclear receptor assays are to quantitatively assess the bioactivity of a test compound as an agonist (activator) or antagonist (inhibition of an agonist response) of a given receptor. Service assays include a positive control reference compound and ‘vehicle’ control for every experiment. A formal study report and all data files are provided to the client upon completion of the study. To receive a quote for your proposed study, complete & submit the online “Request a Quote” form or contact an INDIGO Customer Service Representative to discuss your desired study parameters.
Background
Although technically not a member of the Nuclear Receptor (NR) superfamily, the AhR shares many of the same attributes. The AhR is a member of the basic helix-loop-helix, Per-Arnt-Sim (bHLH-PAS) family of transcription factors and is responsible for the toxicologic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, often referred to as simply “dioxin”) and several other related polycyclic aromatic hydrocarbons. The basic mechanism of action of dioxin and related compounds has been extensively studied, in particular as it relates to regulation of cytochrome P450 1A1 (CYP1A1).
AhR is present in most cell types and in the unactivated state is cytosolic and exists in a complex with chaperone proteins such as heat shock protein 90 (Hsp90). Binding of TCDD and related molecules to AhR leads to nuclear translocation and heterodimerization with its partner protein ARNT, another bHLH-PAS family member. The AhR-ARNT heterodimer binds to specific cognate DNA sequence elements known as dioxin/xenobiotic response elements (DRE/XRE) present in the regulatory region of specific genes such as CYP1A1. Binding of the AhR:ARNT heterodimer to these elements, and subsequent recruitment of transcription coactivator complexes, leads to increased transcription of the specific gene, known as “target genes.” There is a battery of genes affected in this manner and targets include certain xenobiotic-metabolizing enzymes, such as CYP1A1,CYP1A2, CYP2B1, and UGT1A6. In addition, genes affected directly and indirectly by the TCDD/AhR-complex code for both inhibitory and stimulatory growth factors and their gene products affect cellular growth and differentiation leading to tumor promotion and carcinogenicity as well as other forms of toxicity.
INDIGO’s Aryl Hydrocarbon Receptor (AhR) Assay is a cell-based genetic reporter assay that utilizes the luciferase reporter gene Aryl Hydrocarbon Receptor Agonist and Antagonist Assays functionally linked to an AhR-responsive promoter. Thus, quantifying changes in luciferase expression in the treated reporter cells provides a sensitive surrogate measure of the changes in AhR activity.
The principle application of this reporter assay system is in the screening of test samples to quantify any functional activity, either agonist or antagonist, that they may exert against human AhR.
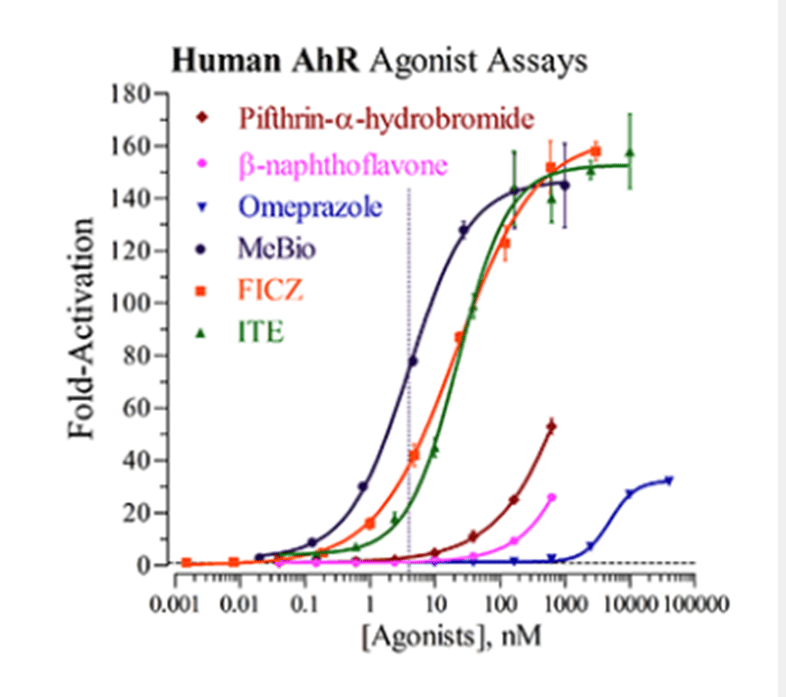
Agonist dose-response analyses of Human AhR.
Agonist analyses of Human AhR Reporter Cells were performed according to the protocol described in the assay technical manual, using the reference agonists MeBIO (provided), FICZ (6-Formylindolo(3,2-b)carbazole; Enzo), ITE (2-(1H-indole-3-ylcarbonyl)-4-thiazolecarboxylic methyl ester; Tocris), β-Napthoflavone (Sigma), Omeprazole and Pifthrin-a-hydrobromide (each from Tocris). Average relative light units (RLU) and corresponding standard deviation (SD) values were determined for each treatment concentration (n ≥ 6). Fold-activation (i.e., S/B) and Z’ values were calculated as described by Zhang, et al. (1999). Least squares fit non-linear regression and EC50 analyses were performed using GraphPad Prism software.
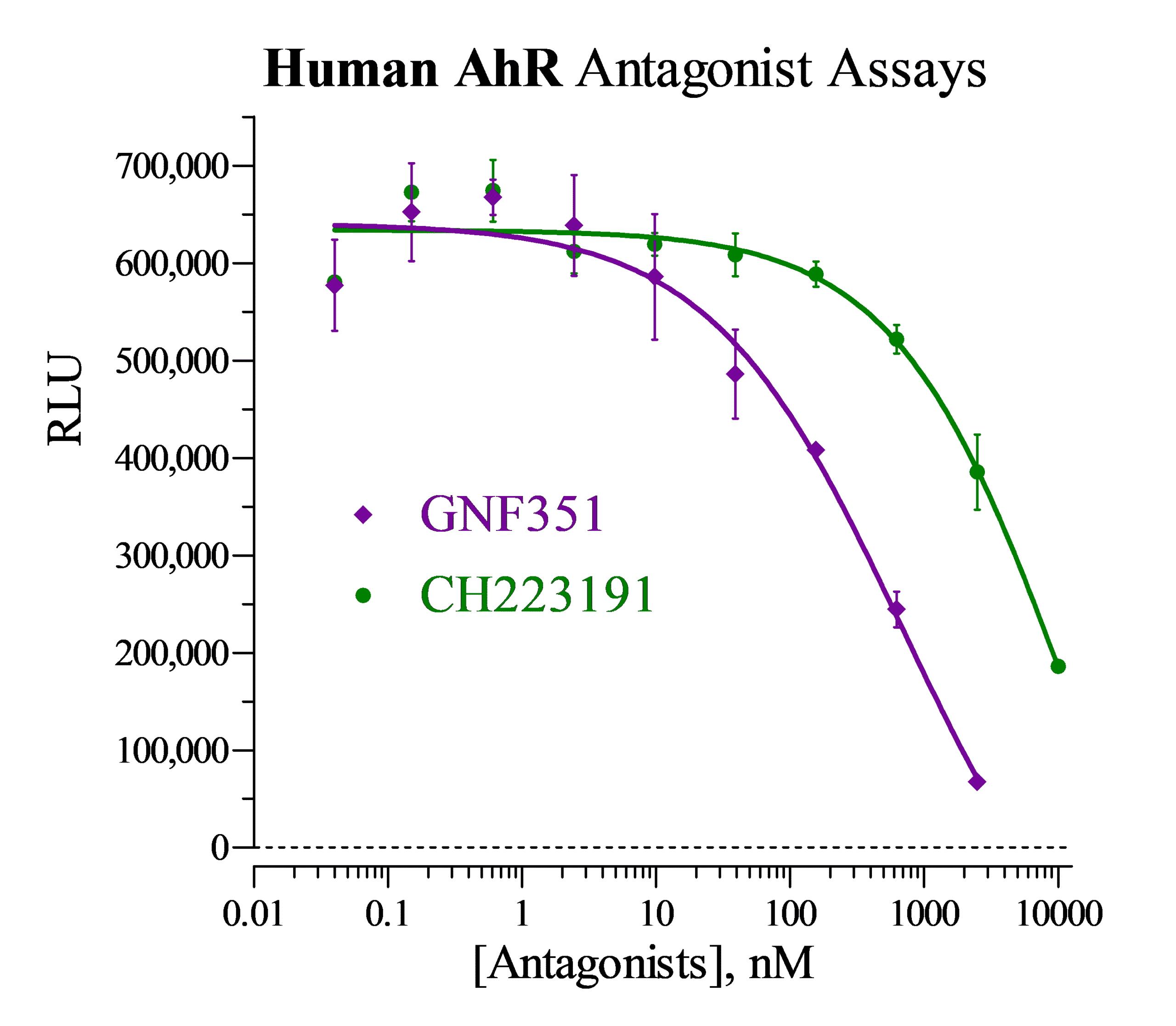
Antagonist dose-response analyses of Human AhR.
Antagonist analyses of Human AhR Reporter Cells were performed according to the protocol described in the assay technical manual, using the reference antagonists GNF351 (Calbiochem) and CH 223191 (Tocris).
1 Review
-
perfect
Everything is perfect of gentaur from packaging to delivery. Highly recommended!






