Description
RORgamma (human) Reporter Assay Kit | IB-IB04001
Product information "RORgamma (human) Reporter Assay Kit"
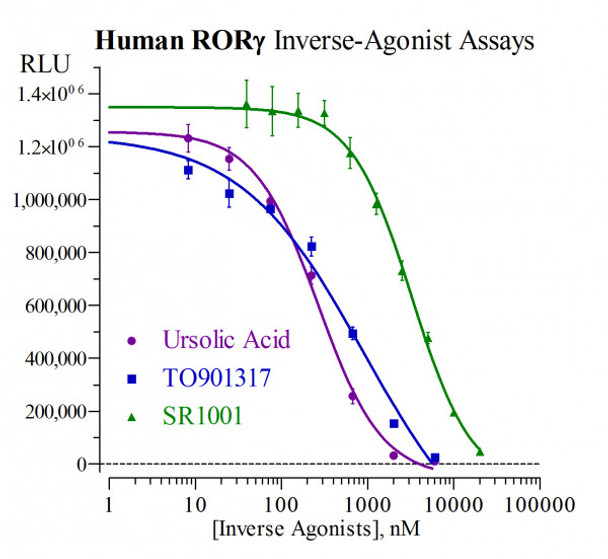
This human RAR-related orphan receptor gamma (RORgamma) mRNA is expressed from the RORC gene in two forms via alternate usage of tissue-specific promoters. Variant 1 mRNA is expressed in numerous tissues, and encodes receptor isoform 1, referred to as RORgamma. Variant 2 mRNA comprises an alternate exon 1 that replaces the exon 1 and 2 sequences found in the Variant 1 transcript. Consequently, variant 2 mRNA presents a unique 5'UTR and modified N-terminal ORF sequences, resulting in the expression of a shorter isoform 2 receptor. The isoform 2 receptor is expressed predominantly in specialized immune cells developing within the thymus, as such it is referred to as RORgammat. This nuclear receptor assay system utilizes proprietary human cells engineered to provide high-level expression of a hybrid form of the human RAR-related orphan receptor gamma. The N-terminal DNA binding domains (DBD) of the native RORgamma and RORgammat receptors have been substituted with that of the yeast GAL4-DBD. Hence, the GAL4- RORgamma hybrid receptor expressed in these reporter cells will not discern any functional differences that may exist between the native isoform 1 and isoform 2 receptors. As is true in vivo, uncharacterized molecular activators present in these reporter cells maintain RORgamma in a constant state of high-level activity. The principal application of this reporter assay system is in the screening of test samples to quantify inverse-agonist activities that they may exert against human RORgamma. Reporter cells are prepared using INDIGO's proprietary CryoMite(TM) process. This cryopreservation method yields high cell viability post-thaw, and provides the convenience of immediately dispensing healthy, division-competent reporter cells into assay plates. There is no need for intermediate spin-and-wash steps, viability determinations, or cell titer adjustments.