Description
Thyroid Hormone Receptor Alpha (TRα; NR1A1) | IB01001
This Thyroid Hormone Receptor Alpha (TRα) assay kit is an all-inclusive firefly luciferase reporter assay system that includes, in addition to TRα Reporter Cells, two optimized media for use during cell culture and (optionally) in diluting the test samples, a reference agonist, Luciferase Detection Reagent, a cell culture-ready assay plate, and a detailed protocol.
TRα Reporter Cells are prepared using INDIGO’s proprietary CryoMite™ process. This cryo-preservation method yields high cell viability post-thaw, and provides the convenience of immediately dispensing healthy, division-competent reporter cells into assay plates. There is no need for intermediate spin-and-wash steps, viability determinations, or cell titer adjustments.
INDIGO’s TR alpha reporter assay kits feature a luciferase detection reagent specially formulated to provide stable light emission between 5 and 90+ minutes after initiating the luciferase reaction. Incorporating a 5-minute reaction-rest period ensures that light emission profiles attain maximal stability, thereby allowing assay plates to be processed in batch. By doing so, the signal output from all sample wells, from one plate to the next, may be directly compared within an experimental set.
TR alpha assay kits are offered in different assay formats to accommodate researchers’ needs: 3x 32, 1x 96, and 1x 384 assay formats for screening small numbers of test compounds, as well as custom bulk reagents for HTS applications.
Assay Kit & Platforms
Bulk assay reagents can be custom manufactured to accommodate any scale of HTS. Please inquire.
Assay Services
The primary application of INDIGO’s cell-based nuclear receptor assays are to quantitatively assess the bioactivity of a test compound as an agonist (activator) or antagonist (inhibition of an agonist response) of a given receptor. Service assays include a positive control reference compound and ‘vehicle’ control for every experiment. A formal study report and all data files are provided to the client upon completion of the study. To receive a quote for your proposed study, complete & submit the online “Request a Quote” form or contact an INDIGO Customer Service Representative to discuss your desired study parameters.
Background
Thyroid hormone receptor alpha (TR-alpha) (erythroblastic leukemia viral (v-erb-a) oncogene homolog, avian), also known as NR1A1, is nuclear receptor protein encoded by the THRA gene. The protein encoded by this gene is a nuclear hormone receptor for triiodothyronine. It is one of the several receptors for thyroid hormone, and has been shown to mediate the biological activities of thyroid hormone. Knockout studies in mice suggest that the different receptors, while having certain extent of redundancy, may mediate different functions of thyroid hormone. Alternatively spliced transcript variants encoding distinct isoforms have been reported.
The Human TRα Reporter Assay Systems utilize proprietary human cells engineered to provide constitutive, high-level expression of Human Thyroid Hormone Receptor Alpha (NR1A1), a ligand-dependent transcription factor commonly referred to as TRα. Additionally, these cells contain a TRα-responsive luciferase reporter gene. Thus, quantifying luciferase activity provides a surrogate measure of TRα activity in the treated reporter cells.
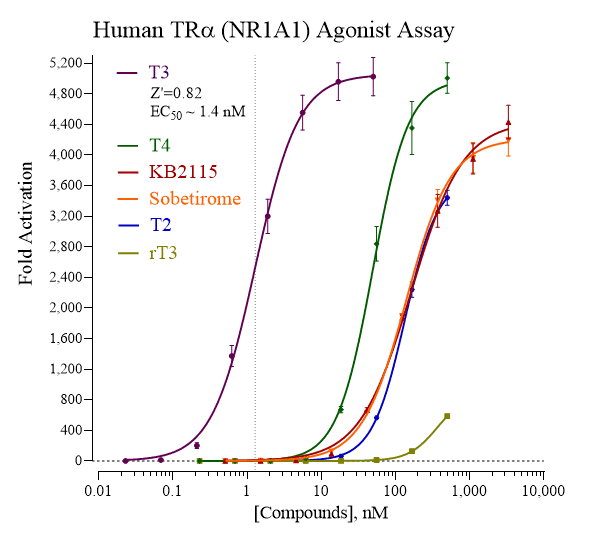
Activity dose-response of TRα using various reference agonists. Dose-response analyses of TRα were performed according to the protocol provided in this Technical Manual. Reporter Cells were treated with T3 (3,3’,5-L-Triiodothyronine), T2 (3,3’-L-Triiodothyronine), rT3 (3,3’,5’-L-Triiodothyronine), T4 (L-Thyroxine), KB2115, and Sobetirome. Appendix 1 in the assay technical manual provides a recommended range of treatment concentrations for T3, the reference agonist provided with this kit. Luminescence per assay well was quantified and values of average relative light units (RLU) and corresponding standard deviation (SD) were determined for each treatment concentration (n ≥ 4). Fold-activation (signal-to-background) and Z’ values were calculated as described by Zhang, et al. (1999). Non-linear regression analyses and EC50 calculations were performed using GraphPad Prism software.