Description
DNAzol® GENOMIC DNA ISOLATION REAGENT
DNAzol® is a complete and ready to use reagent for the isolation of genomic DNA from solid and liquid samples of animal
and plant origin. The DNAzol procedure is based on the use of a novel guanidine-detergent lysing solution that hydrolyzes RNA
and allows the selective precipitation of DNA from a cell lysate. Developed by P. Chomczynski (1), DNAzol is a patented DNA
isolation method (U.S. patent no. 5, 945, 515) that combines both reliability and efficiency with simplicity of the isolation protocol.
The DNAzol protocol is fast and permits isolation of genomic DNA from a large number of samples of small or large volumes (5).
During the isolation, a biological sample is lysed or homogenized in DNAzol and the genomic DNA is precipitated from
the lysate with ethanol. Following an ethanol wash, DNA is solubilized in water or 8 mM NaOH. The procedure can be completed
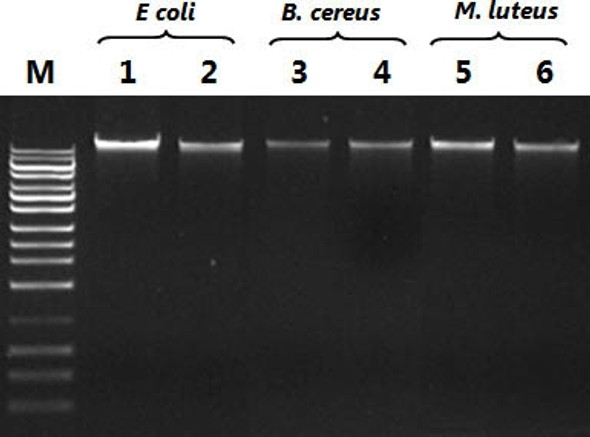
in 10 - 30 minutes with a genomic DNA recovery of 70 - 100%. The isolated DNA can be used, without additional purification,
for Southern analysis, dot blot hybridization, molecular cloning, PCR and other molecular biology and biotechnology applications.
STABILITY: DNAzol is stable at room temperature for at least two years after the date of purchase.
HANDLING PRECAUTIONS: DNAzol contains irritants. Handle with care, avoid contact with skin, use eye protection (shield,
safety goggles). In case of contact, wash skin with a copious amount of water. Seek medical attention.
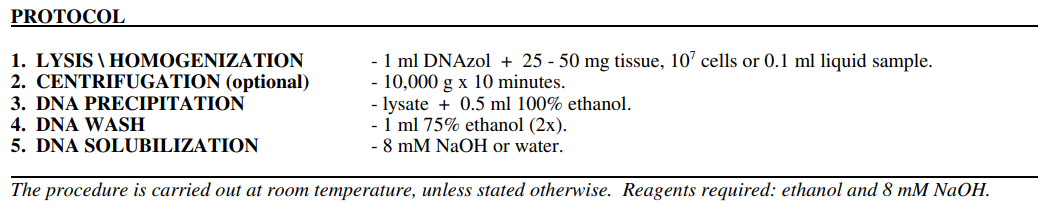
1. LYSIS / HOMOGENIZATION
A. TISSUES. Homogenize tissues in a hand held glass-Teflon homogenizer. Use a loosely fitting homogenizer, with a tolerance
greater than 0.1 - 0.15 mm. Homogenize 25 - 50 mg tissue in 1 ml of DNAzol by applying as few strokes as possible. Typically,
5 - 10 strokes are required for complete homogenization. Small amounts (5 - 10 mg) of soft tissues, such as spleen or brain can be
dispersed and lysed by repetitive pipetting with a micropipette. Store the homogenate for 5 - 10 minutes at room temperature.
B. CELLS. Cells grown in monolayer should be lysed directly in a culture dish. Pour off media, add DNAzol and pass the cell
lysate several times through a pipette. Add 0.75 - 1.0 ml of DNAzol per 10 cm2
culture plate area.
Cell pellets or suspensions, add 1 ml of DNAzol to 107
cells (volume < 0.1 ml) and lyse the cells by repeated pipetting.
Cell nuclei, add 1 ml of DNAzol to 1 - 3 x 107
cell nuclei (volume < 0.1 ml) and lyse the nuclei by inversion or repeated pipetting.
To minimize shearing the DNA molecules, mix DNA solutions by inversion; avoid vigorous shaking or vortexing.
Please see Note 5 for a description of an optional proteinase K digestion procedure.
2. CENTRIFUGATION (Optional)
Sediment the homogenate for 10 minutes at 10,000 g at 4 - 25 C. Following centrifugation, transfer the resulting viscous
supernatant to a fresh tube.
This step removes insoluble tissue fragments, partially hydrolyzed RNA and excess polysaccharides from the lysate/homogenate.
It is required only for the isolation of DNA from tissues such as liver, muscles and most plant tissues containing a large amount
of cellular and/or extracellular material and is also recommended for the isolation of RNA-free DNA.
3. DNA PRECIPITATION
Precipitate DNA from the lysate/homogenate by the addition of 0.5 ml of 100% ethanol per 1 ml of DNAzol used for the
isolation. Mix samples by inverting tubes 5 - 8 times and store at room temperature for 1 - 3 minutes. Make sure that DNAzol and
ethanol mix well to form a homogenous solution. DNA should quickly become visible as a cloudy precipitate. Remove the DNA
precipitate by spooling with a pipette tip. Swirl the DNA onto the tip and attach it to the tube wall near the top of the tube by gently
sliding the DNA off the tip. Alternatively, transfer the DNA to a clean tube. Store the tubes upright for about 1 minute and remove
from the bottom of the tubes the remaining lysate/homogenate.
Degraded DNA and small quantities of DNA (< 15 µg) do not spool onto a pipette tip. In this case, sediment the precipitated DNA
by centrifugation at 5,000 g for 5 minutes at 4 - 25 C.
4. DNA WASH
Wash the DNA precipitate twice with 0.8 - 1.0 ml of 75% ethanol. At each wash, suspend the DNA in ethanol by inverting
the tubes 3 - 6 times. Store the tubes vertically for 0.5 - 1 minutes to allow the DNA to settle to the bottom of the tubes and remove
ethanol by pipetting or decanting.
If necessary, sediment the DNA pellet at 1,000 g for 1 - 2 min at 4 - 25 C. To further remove contaminants when isolating DNA
from tissues, the first ethanol wash can be replaced with wash in a solution containing 70% DNAzol and 30% ethanol.