High Sensitivity Kanamycin ELISA Test Kit | DEIA-WZ048V
- SKU:
- DEIA-WZ048V
- Availability:
- 5 Working days
- Sample:
- biological samples
- Species Reactivity:
- N/A
- Storage:
- Store the kit at 2-8°C
Description
Intended Use
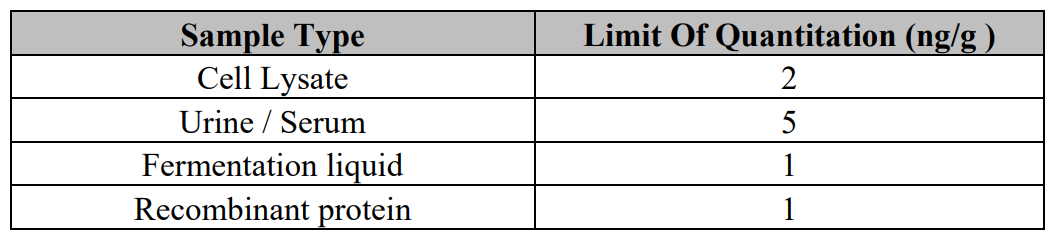
High Sensitivity Kanamycin ELISA Test Kit is a competitive enzyme immunoassay for the quantitative analysis
of Kanamycin in biological samples (cell lysate, urine, serum, recombinant protein, and fermentation liquid, etc.).
The unique features of the kit are:
1. High recovery (>80%), rapid (10-40 minutes), and cost-effective extraction methods.
2. Detection limit is 0.2 ng/g.
3. High reproducibility.
4. A quick ELISA assay (less than 1 hour regardless of number of samples).
General Description
Kanamycin or kanamycin A is an aminoglycoside bactericidal antibiotic, used to treat a wide variety of infections and
tuberculosis. Kanamycin is isolated from the bacterium Streptomyces kanamyceticus and its most commonly used form
is kanamycin sulfate. Kanamycin is commonly used as an antibiotic during the cell culture process. Regulatory
authorities across the world have restricted and sought to quantify the Kanamycin residue in view of its potent action on
human beings in the in-process and finished pharmaceutical products. Kanamycin residue in the production of biological
products may lead to abnormal reactions of human beings, thus strict MRLs have been established. This kit is a rapid
test product for the determination of kanamycin residues which is sensitive, accurate and time-saving. It can
considerably reduce the operation errors in the assay.
Principles of Testing
The method is based on a competitive colorimetric ELISA assay. The Kanamycin-BSA has been coated in the plate
wells. During the analysis, sample and Kanamycin antibody (Antibody #1) and HRP-Conjugate (HRP-Conjugated
Antibody #2) are added to the wells for incubation. If the Kanamycin residue is present in the sample, it will compete
with the Kanamycin on the plate wells for the Kanamycin antibody. The secondary antibody, tagged with a peroxidase
enzyme, targets the primary antibody that is complexed to the drug coated on the plate wells. The resulting color
intensity, after addition of the HRP substrate (TMB), has an inverse relationship with the Kanamycin residue
concentration in the sample.
Reagents And Materials Provided
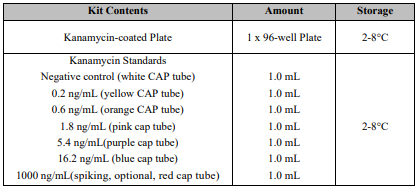
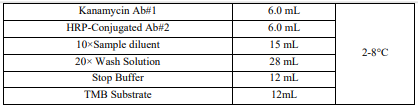
*Kanamycin ELISA Test Kit has the capacity for 96 determinations or testing of 42 samples in duplicate (assuming 12
wells for standards).
Materials Required But Not Supplied
1. Microtiter plate reader (450 nm)
2. Incubator
3. Vortex mixer
4. 10, 20, 100 and 1000 μL pipettes
5. Multi-channel pipette: 50-300 μL (Optional)
Storage
Store the kit at 2-8°C. The shelf life is 12 months when the kit is properly stored.
*If you are not planning to use the kit for over 3 months, store Kanamycin Antibody #1 and HRP-Conjugated Antibody
#2 at -20°C or in a freezer.
*Return any unused microwells to the foil bag and reseal them with the desiccant provided in the original package.
Specimen Collection And Preparation
The kit was validated for cell lysate, urine, serum, recombinant protein, and fermentation liquid. In principle other
sample types than these are also suitable but have to be tested in advance. For more details please contact your local
supplier or the manufacturer directly.
Be sure samples are properly stored. In general, samples should be refrigerated at 2-4°C for no more than 1-2 days.
Freeze samples to a minimum of -20°C if they need to be stored for a longer period. Frozen samples can be thawed
at room temperature (20 – 25°C / 68 – 77°F) or in a refrigerator before use.
Preparation of 1X Sample Dilution Buffer
Mix 1 volume of the 10X Sample Dilution Buffer with 9 volumes of distilled water.
Cell Lysate
1. Take 100 μL of cell lysate, add 900 μL of 1×Sample diluent Buffer, mix well.
2. Centrifuge for 5 minutes at 4000 x g.
3. Take 50 μL of the supernatant per well for the assay.
Note: Dilution factor: 10.
Urine/Serum
1. Take 50 μL of urine or serum sample, add 2 mL of 1X Sample diluent , mix well.
2. Centrifuge for 5 minutes at 4,000 x g.
3. Take 50 μL of the supernatant per well for the assay.
Note: Dilution factor: 25.
Recombinant protein, Fermentation liquid
1. Centrifuge 1 mL sample at 4,000 x g for 5 minutes.
2. Get the supernatant 50 μL sample to 200 μL 1X Sample diluent.
3. Use 50 μL per well for the assay
Note: Dilution factor: 5. If needed, the sample can be further diluted with 1X Sample diluent.
Reagent Preparation
IMPORTANT: All reagents should be brought up to room temperature before use (1 – 2 hours at 20 – 25°C / 68 –
77°F); Make sure you read “Precautions” section. Solutions should be prepared just prior to ELISA test. All reagents
should be mixed by gently inverting or swirling prior to use. Prepare volumes that are needed for the number of wells
being run. Do not return the reagents to the original stock tubes/bottles. Using disposable reservoirs when handling
reagents is recommended which can minimize the risk of contamination.
Preparation of 1X Wash Solution
Mix 1 volume of the 20X Wash Solution with 19 volumes of distilled water.
Assay Procedure
Label the individual strips that will be used and aliquot reagents as the following example:
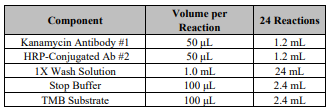
1. Add 50 μL of each Kanamycin Standards in duplicate into different wells (Add standards to plate only in the order
from low concentration to high concentration).
2. Add 50 μL of each sample in duplicate into different sample wells.
3. Add 50 μL of HRP-Conjugated Ab#2 to each well and 50 μL of Kanamycin Antibody #1 to each well. Mix well by
gently rocking the plate manually for 30s.
4. Incubate the plate for 30 minutes at room temperature (20 – 25°C / 68 – 77°F). (Avoid direct sunlight and cold bench
tops during the incubation. Cover the microtiter plate while incubating is recommended).
5. Wash the plate 4 times with 250 μL of 1X Wash Solution. After the last wash, invert the plate and gently tap the plate
dry on paper towels (Perform the next step immediately after plate washings. Do not allow the plate to air dry
between working steps).
6. Add 100 μL of TMB substrate. Time the reaction immediately after adding the substrate. Mix the solution by gently
rocking the plate manually for 30s while incubating (Do not put any substrate back to the original container to avoid
any potential contamination. Any substrate solution exhibiting coloration is indicative of deterioration and should be
discarded. Covering the microtiter plate while incubating is recommended).
7. After incubating for 15 minutes at room temperature (20 – 25°C / 68 – 77°F), add 100 μL of Stop Buffer to stop the
enzyme reaction.
8. Read the plate as soon as possible following the addition of Stop Buffer on a plate reader with 450 nm wavelength
(Before reading, use a lint-free wipe on the bottom of the plate to ensure no moisture or fingerprints interfere with the
readings).
Calculation
A standard curve can be constructed by plotting the mean relative absorbance (%) obtained from each reference standard
against its concentration in ng/mL on a Logarithm curve.
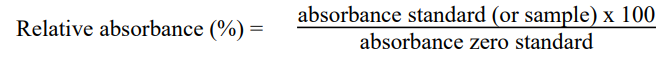
When use computing software, recommends Log/Log standard curves.
Typical Standard Curve
This curve cannot be used for the assay calculations and is listed for quality control purpose only. New standard curve
must be generated each time the assay is run.
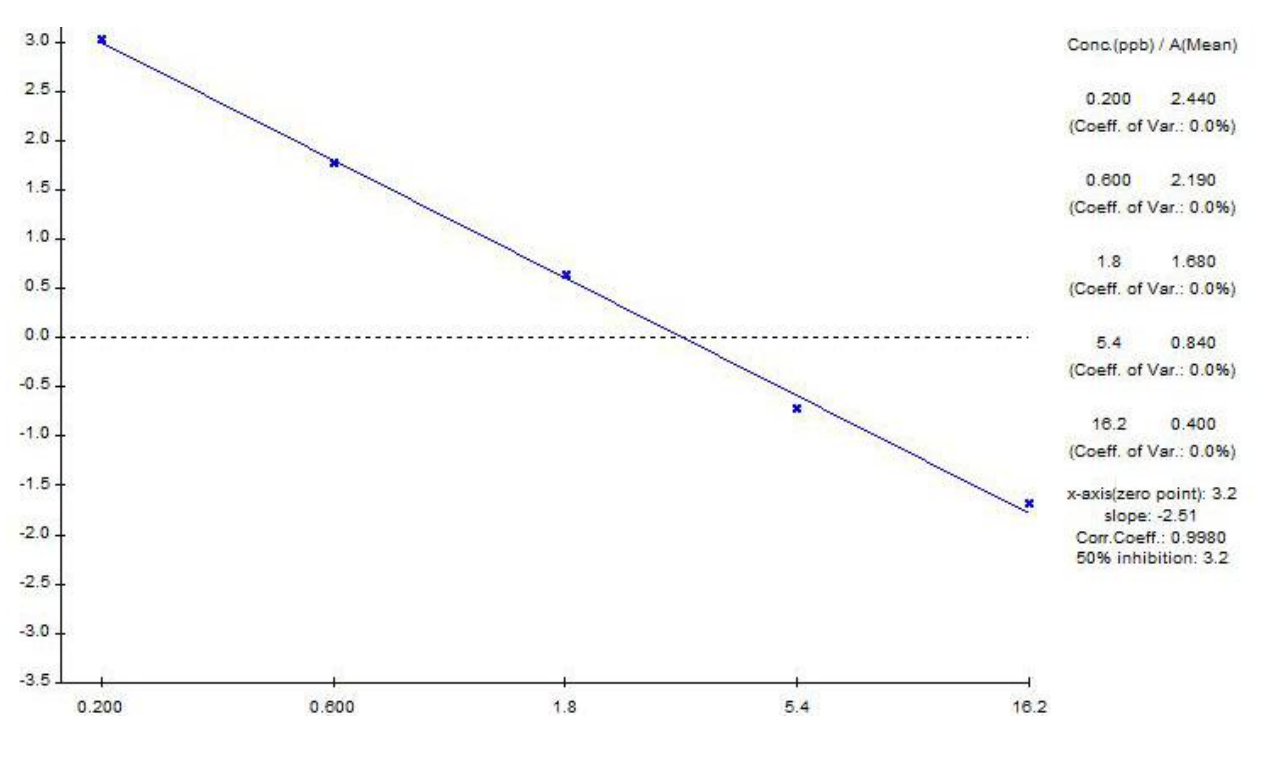
Detection Range
The standards / linear graph range is 0.2-16.2 ng/mL.
Detection Limit