Description
I. Introduction:
Kanamycin is an aminoglycoside antibiotic and is widely used in treating animal diseases. It harms the 8th cranial nerves, causing
damages to the vestibular and cochlear. The main manifestations of renal toxicity are the damages of the proximal convoluted tubule,
causing protein urine, hematuresis, renal hypofunction, etc. The residues of Kanamycin in animal derived food affect human health, in
China and Occident, Kanamycin has been limited to use for its neurotoxicity and nephrotoxicity. This kit is a detection product developed
based on competitive ELISA technology, with operation time as short as 50 min and a sensitivity of 0.5 ppb, and linear range from 0.5 ppb
~40.5 ppb.
II. Application:
This ELISA kit is used for in vitro quantitative determination of Kanamycin
Detection Range: 0.5 – 40.5 ppb
Sensitivity: 0.5 ppb
Detection limitation: 5 ppb for milk and tissue, and 15 ppb milk powder
III. Sample Type:
Tissue, Milk and milk powder
IV. Kit Contents:
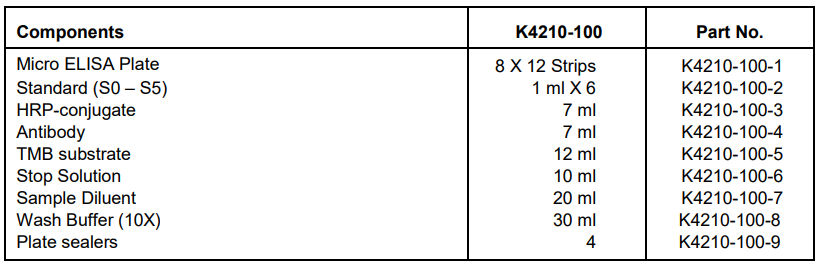
V. User Supplied Reagents and Equipment:
Chemicals: Na2HPO4∙12H2O, NaH2PO4∙2H2O, and Trichloroacetic acid (TCA)
Microplate reader capable of measuring absorbance at 450 nm
25°C incubator
Precision pipettes with disposable tips
Distilled or deionized water
Clean eppendorf tubes and graduated cylinders for preparing standards or sample dilutions
Absorbent paper
VI. Storage and Handling:
The entire kit may be stored at 4°C for up to 12 months from the date of shipment. Opened kit may be stable for 1 month at 4°C.
VII. Reagent and Sample Preparation:
Note: Bring all reagents to room temperature (20-25°C) 30 minutes before use.
Before using the kit, spin tubes and bring down all components to the bottom of tubes.
1. Extraction Solution 1: Weigh 5.37 g of Na2HPO4∙12H2O and 0.78 g of NaH2PO4∙2H2O to 100 ml of deionized water, mix thoroughly.
2. Extraction Solution 2: Dilute 5 ml of Sample Diluent into 95 ml deionized or distilled water, mix well.
3. Extraction Solution 3: Dilute 3 g of TCA into 100 ml deionized or distilled water, shake well
4. Wash Buffer (1X): If crystals have formed in the concentrate, warm up to room temperature and mix gently until the crystals have
completely dissolved. Dilute 10 mL of Wash Buffer (10X) into 90 mL deionized or distilled water to prepare 100 ml of Wash Buffer
(1X). Keep it at 4°C for one month.
5. Standards Concentration:

6. Sample Preparation:
Note: The prepared sample maybe stored for up to one day at 2-8°C.
A. Milk and fresh milk
1. Bring the milk sample to room temperature
2. Transfer 100 μL of sample into a new centrifugal tube and add 900 μL of Extraction Solution 1, shake well.
3. Take 50 μL of sample for further analysis. (Dilution factor: 10)
B. Milk power
1. Weigh 1 g of milk power sample, add 5ml of Extraction Solution 2, shake properly for 5 min.
2. Add 4 ml of Extraction Solution 3, shake properly for 5 min. Centrifuge at 4000 rpm for 10 min.
3. Transfer 100 μl of supernatant into a new centrifugal tube, add 200 μl of Sample Diluent, shake well.
4. Take 50 μLof sample for further analysis. (Dilution factor: 30)
C. Tissue (chicken, pork)
1. Weigh 1 g of the homogenized tissue sample, add 10 ml of Extraction Solution 1, shake properly for 5 min. Centrifuge at 4000 rpm for
10 min.
2. Take 50 μl of supernatant sample for further analysis. (Dilution factor: 10)
VIII. Assay Protocol:
Note: Bring all reagents and samples to room temperature 30 minutes prior to the assay.
It is recommended that all standards and samples be run at least in duplicate.
A standard curve must be run with each assay.
1. Prepare all reagents, samples and standards as instructed in section VII.
2. Add 50 μl of Standard or Sample per well. Then add 50 μl of HRP-conjugate to each well and 50 μl of Antibody to each well. Cover
the microtiter plate with a new adhesive strip and mix well, then incubate for 30 min at 25°C.
3. Aspirate each well and wash, repeating the process 4 times. Wash by filling each well with 250 μl of Wash Buffer using a squirt bottle,
multi-channel pipette, manifold dispenser, or autowasher, and let it stand for 30 seconds, complete removal of liquid at each step is
essential to good performance.
4. Add 100 μl of TMB Substrate to each well, mix well. Incubate for 15 minutes at 25°C. Protect from light.
5. Add 50 μl of Stop Solution to each well, gently tap the plate to ensure thorough mixing.
6. Read result at 450 nm within 10 minutes.
IX. CALCULATION:
The mean values of the absorbance values obtained for the standards and the samples are divided by the absorbance value of the first
standard (zero standard) and multiplied by 100%. The zero standard is thus made equal to 100% and the absorbance values are quoted in
percentages.
Absorbance Value (%) = B/B0 X 100%
B: The average absorbance value of the sample or standard
B0 : The average absorbance value of the 0 ppb standard
To draw a standard curve: Take the absorbency value of standards as y-axis, logarithmic of the concentration of the Kanamycin standards
solution (ppb) as x-axis. The Kanamycin concentration of each sample (ppb), which can be read from the calibration curve, is multiplied by
the corresponding dilution factor of each sample followed, and the actual concentration of sample is obtained.






