Description
Human Troponin I, Slow Skeletal Muscle (TNNI1) ELISA Kit | CSB-EL024011HU
This assay employs the quantitative sandwich enzyme immunoassay technique.
Antibody specific for TNNI1 has been pre-coated onto a microplate. Standards
and samples are pipetted into the wells and any TNNI1 present is bound by the
immobilized antibody. After removing any unbound substances, a
biotin-conjugated antibody specific for TNNI1 is added to the wells. After
washing, avidin conjugated Horseradish Peroxidase (HRP) is added to the wells.
Following a wash to remove any unbound avidin-enzyme reagent, a substrate
solution is added to the wells and color develops in proportion to the amount of
TNNI1 bound in the initial step. The color development is stopped and the
intensity of the color is measured.
DETECTION RANGE
0.312 ng/ml-20 ng/ml.
SENSITIVITY
The minimum detectable dose of human TNNI1 is typically less than 0.078
ng/ml.
The sensitivity of this assay, or Lower Limit of Detection (LLD) was defined as
the lowest protein concentration that could be differentiated from zero. It was
determined the mean O.D value of 20 replicates of the zero standard added by
their three standard deviations.
SPECIFICITY
This assay has high sensitivity and excellent specificity for detection of human
TNNI1. No significant cross-reactivity or interference between human TNNI1 and
analogues was observed.
Note: Limited by current skills and knowledge, it is impossible for us to complete
the cross-reactivity detection between human TNNI1 and all the analogues,
therefore, cross reaction may still exist.
3
PRECISION
Intra-assay Precision (Precision within an assay): CV%<8%
Three samples of known concentration were tested twenty times on one plate to assess.
Inter-assay Precision (Precision between assays): CV%<10% Three samples of known concentration were tested in twenty assays to assess.
LIMITATIONS OF THE PROCEDURE
FOR RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC
PROCEDURES.
The kit should not be used beyond the expiration date on the kit label.
Do not mix or substitute reagents with those from other lots or sources.
If samples generate values higher than the highest standard, dilute the
samples with Sample Diluent and repeat the assay.
Any variation in Sample Diluent, operator, pipetting technique, washing
technique, incubation time or temperature, and kit age can cause variation
in binding.
This assay is designed to eliminate interference by soluble receptors,
binding proteins, and other factors present in biological samples. Until all
factors have been tested in the Immunoassay, the possibility of
interference cannot be excluded.
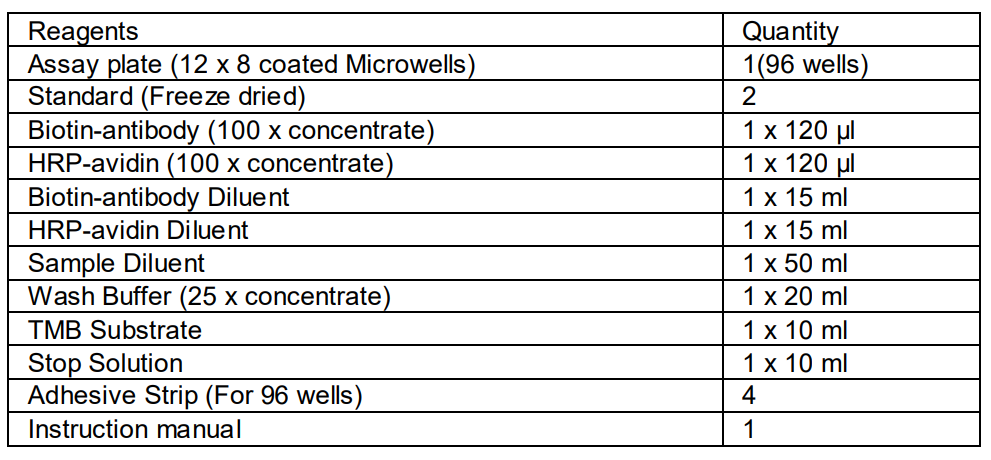
MATERIALS PROVIDED
STORAGE