Description
MMQCI Unyvero LRT/Pneumonia Control Panel M404 Part Number: M404
Kit Contains: 12 tubes x 180µL
4 each of Positive A, Positive B & Negative
Unyvero LRT/Pneumonia Control Panel M404 DataSheet
INTENDED USE:
Unyvero LRT/Pneumonia Control Panel M404 is intended for use as an external positive and negative quality control to monitor the performance of in vitro laboratory nucleic acid testing procedures for the qualitative detection of bacterial targets; Acinetobacter spp., Chlamydia pneumoniae, Citrobacter freundii, Enterobacter cloacae complex, Enterobacter aerogenes†, Escherichia coli, Haemophilus influenzae, Klebsiella oxytoca, Klebsiella pneumoniae, Klebsiella variicola, Legionella pneumophila, Moraxella catarrhalis, Morganella morganii, Mycoplasma pneumoniae, Pneumocystis jirovecii†, Proteus spp., Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus, Stenotrophomonas maltophilia, Streptococcus pneumoniae, and antibiotic resistance markers; ctx-M, imp†, kpc, mecA, mecC†, ermB†, ndm, oxa-23, oxa-24, oxa-48, oxa-58, vim, tem, sul1†, shv†, gyrA83/87† (E.coli and P.aeruginosa) by the Unyvero Lower Respiratory Tract (LRT) Application and Unyvero Hospitalized Pneumonia (HPN) Application performed on the Unyvero System. Unyvero LRT/Pneumonia Control Panel M404 is composed of synthetic DNA specifically designed for and intended to be used with the Unyvero LRT and Unyvero HPN Applications.
†Target detected with Unyvero HPN Application only, not available in U.S.
PRODUCT SUMMARY and PRINCIPLE:
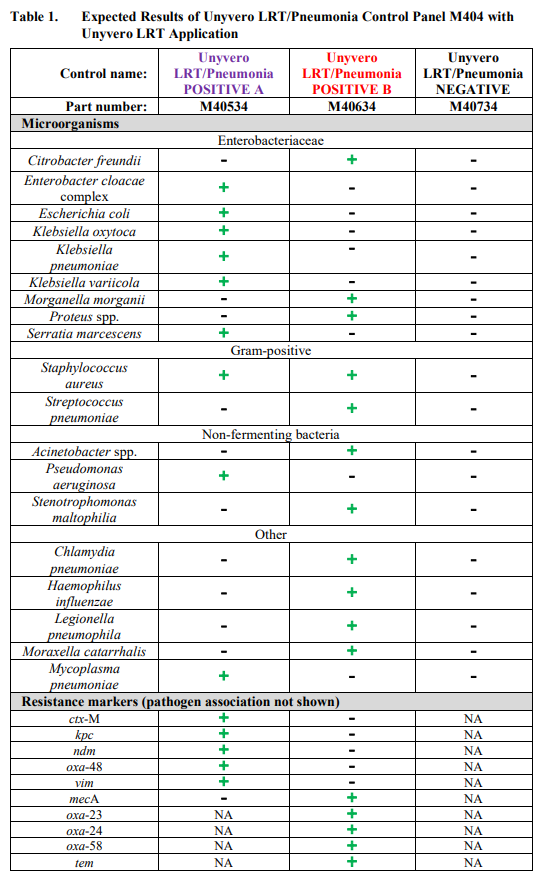
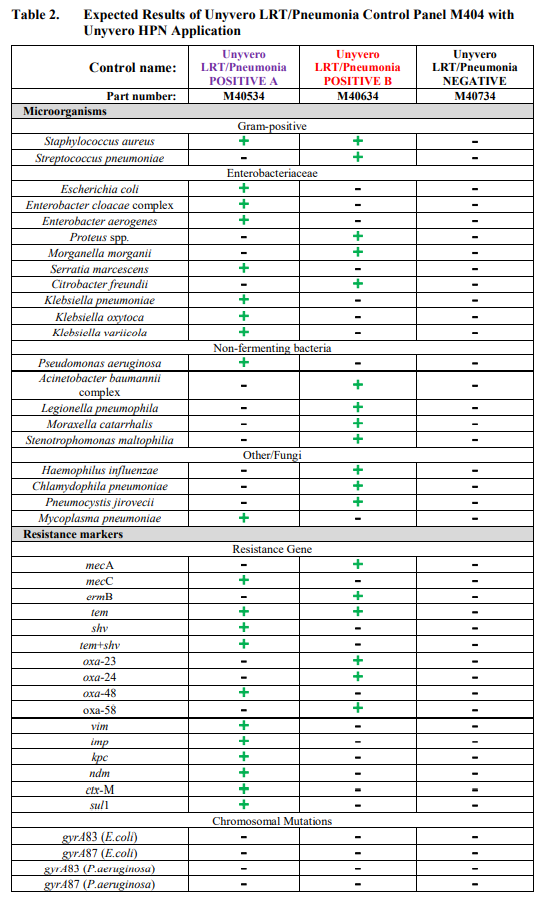
Unyvero LRT/Pneumonia Control Panel M404 is composed of 3 controls, Unyvero LRT/Pneumonia Positive A, Unyvero LRT/Pneumonia Positive B and Unyvero LRT/Pneumonia Negative. Unyvero LRT/Pneumonia Positive A and Positive B contain surrogate control material composed of synthetic DNA corresponding to genome segments of pathogens and associated antibiotic resistance markers listed in Table 1 (page 1) and Table 2 (page 2). Unyvero LRT/Pneumonia Negative contains no DNA.
Routine use of quality controls that are consistent lot to lot assists the laboratory in identifying shifts, trends, and increased frequency of random errors caused by variations in the test system, such as failing reagents. Early investigation can prevent failed assay runs.
COMPOSITION:
The Unyvero LRT/Pneumonia Control Panel M404 is comprised of 12 tubes, 4 tubes of each Positive A and B, and 4 tubes of Negative control, 180µL each. Unyvero LRT/Pneumonia Positive controls contain synthetic DNA suspended in a non-infectious solution of buffers, preservatives and stabilizers. Unyvero LRT/Pneumonia Negative control contains buffers and preservatives. Tables 1 and 2 list the pathogens and antibiotic resistance markers that are monitored by the Unyvero LRT/Pneumonia Control Panel M404 when tested by Unyvero LRT (Table 1) and Unyvero HPN (Table 2) Application on the Unyvero System.
PRECAUTIONS, WARNINGS and LIMITATIONS:
- Do not dilute or transfer to another storage
- This product is intended for in vitro analytical testing and is provided for Research Use Only (RUO).
- This product is for use with Unyvero LRT and Unyvero HPN Applications on Unyvero It does not contain the entire genome of pathogens listed in Tables 1 and 2.
- This product is not intended for use as a substitute for the internal controls provided in the Unyvero LRT or Unyvero HPN
- This product does not contain any biological material of human or animal Universal Precautions are NOT required when handling this product.
- Quality control materials should be used in accordance with local, state, federal regulations and accreditation
- Unyvero LRT/Pneumonia Control Panel M404 cannot be cloned, sold, or transferred without the explicit written consent of
INSTRUCTIONS FOR USE:
- Allow the control to be tested to come completely to room temperature (18° – 25°C).
- Use the control as DO NOT DILUTE.
- Follow procedures listed in the Unyvero Instructions for Use provided by
- Immediately before use, mix the control thoroughly by pulse-vortexing for 5-10 Gently tap the tube several times on the bench to remove any liquid caught in the cap before opening the tube.
- Remove the Transport Cap by gently pulling it upward and dispose of it. If necessary, use a new pipette tip to remove any bubbles on the surface of the Unyvero Sample
- Aspirate 180µL of the vortexed Control into the Sample Tube, and close it using the Unyvero Sample Tube Cap provided in the kit, align the small nodules on the neck of the Sample Tube with the openings on the Cap and press down to lock in
- Continue to process according to the Unyvero Instructions for
Notice: Unyvero is a registered trademark of Curetis GmbH, all rights reserved.
STORAGE and STABILITY:
The Unyvero LRT/Pneumonia Control Panel M404 should be stored refrigerated at 2-8°C. Unopened Unyvero LRT/Pneumonia Control Panel M404 material is stable through the expiration date printed on the kit label when consistently stored refrigerated. Unyvero LRT/Pneumonia Control Panel M404 components are for single use. Discard after use according to your local and federal regulations.
EXPECTED VALUES:
The laboratory should follow Good Laboratory Practice (GLP) and establish its own performance characteristics for Unyvero LRT/Pneumonia Control Panel M404 in demonstrating adequate system performance.
Expected results when controls are analyzed on the Unyvero System are listed in Table 1 for the Unyvero LRT Application and Table 2 for the Unyvero HPN Application.
INTENDED USE:
Unyvero LRT/Pneumonia Control Panel M404 is intended for use as an external positive and negative quality control to monitor the performance of in vitro laboratory nucleic acid testing procedures for the qualitative detection of bacterial targets; Acinetobacter baumannii complex, Chlamydophila pneumoniae, Citrobacter freundii, Enterobacter cloacae complex, Enterobacter aerogenes†, Escherichia coli, Haemophilus influenzae, Klebsiella oxytoca, Klebsiella pneumoniae, Klebsiella variicola, Legionella pneumophila, Moraxella catarrhalis, Morganella morganii, Mycoplasma pneumoniae, Pneumocystis jirovecii†, Proteus spp., Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus, Stenotrophomonas maltophilia, Streptococcus pneumoniae, and antibiotic resistance markers; ctx-M, imp†, kpc, mecA, mecC†, ermB†, ndm, oxa-23, oxa-24, oxa-48, oxa-58, vim, tem, sul1†, shv†, gyrA83/87† (E.coli and P.aeruginosa) by the Unyvero Lower Respiratory Tract (LRT) Application and Unyvero Hospitalized Pneumonia (HPN) Application performed on the Unyvero System. Unyvero LRT/Pneumonia Control Panel M404 is composed of synthetic DNA specifically designed for and intended to be used with the Unyvero LRT and Unyvero HPN Applications.
†Target detected with Unyvero HPN Application only, not available in U.S.
PRODUCT SUMMARY and PRINCIPLE:
Unyvero LRT/Pneumonia Control Panel M404 is composed of 3 controls, Unyvero LRT/Pneumonia Positive A, Unyvero LRT/Pneumonia Positive B and Unyvero LRT/Pneumonia Negative. Unyvero LRT/Pneumonia Positive A and Positive B contain surrogate control material composed of synthetic DNA corresponding to genome segments of pathogens and associated antibiotic resistance markers listed in Table 1 (page 1) and Table 2 (page 2). Unyvero LRT/Pneumonia Negative contains no DNA.
Routine use of quality controls that are consistent lot to lot assists the laboratory in identifying shifts, trends, and increased frequency of random errors caused by variations in the test system, such as failing reagents. Early investigation can prevent failed assay runs.
COMPOSITION:
The Unyvero LRT/Pneumonia Control Panel M404 is comprised of 12 tubes, 4 tubes of each Positive A and B, and 4 tubes of Negative control, 180µL each. Unyvero LRT/Pneumonia Positive controls contain synthetic DNA suspended in a non-infectious solution of buffers, preservatives and stabilizers. Unyvero LRT/Pneumonia Negative control contains buffers and preservatives. Tables 1 and 2 list the pathogens and antibiotic resistance markers that are monitored by the Unyvero LRT/Pneumonia Control Panel M404 when tested by Unyvero LRT (Table 1) and Unyvero HPN (Table 2) Applications on the Unyvero System.
PRECAUTIONS, WARNINGS and LIMITATIONS:
- Do not dilute or transfer to another storage
- This product is intended for in vitro analytical testing and is provided for Research Use Only (RUO).
- This product is for use with Unyvero LRT and Unyvero HPN Applications on Unyvero It does not contain the entire genome of pathogens listed in Tables 1 and 2.
- This product is not intended for use as a substitute for the internal controls provided in the Unyvero LRT or Unyvero HPN
- This product does not contain any biological material of human or animal Universal Precautions are NOT required when handling this product.
- Quality control materials should be used in accordance with local, state, federal regulations and accreditation
- Unyvero LRT/Pneumonia Control Panel M404 cannot be cloned, sold, or transferred without the explicit written consent of
INSTRUCTIONS FOR USE:
- Allow the control to be tested to come completely to room temperature (18° – 25°C).
- Use the control as DO NOT DILUTE.
- Follow procedures listed in the Unyvero Instructions for Use provided by
- Immediately before use, mix the control thoroughly by pulse-vortexing for 5-10 seconds. Gently tap the tube several times on the bench to remove any liquid caught in the cap before opening the
- Remove the Transport Cap by gently pulling it upward and dispose of If necessary, use a new pipette tip to remove any bubbles on the surface of the Unyvero Sample Tube.
- Aspirate 180µL of vortexed Control into the Sample Tube, and close it using the Unyvero Sample Tube Cap provided in the kit, align the small nodules on the neck of the Sample Tube with the openings on the Cap and press down to lock in
- Continue to process according to Unyvero Instructions for
Notice: Unyvero is a registered trademark of Curetis GmbH, all rights reserved.
STORAGE and STABILITY:
The Unyvero LRT/Pneumonia Control Panel M404 should be stored refrigerated at 2-8°C. Unopened Unyvero LRT/Pneumonia Control Panel M404 material is stable through the expiration date printed on the kit label when consistently stored refrigerated. Unyvero LRT/Pneumonia Control Panel M404 components are for single use. Discard after use according to your local and federal regulations.
EXPECTED VALUES:
The laboratory should follow Good Laboratory Practice (GLP) and establish its own performance characteristics for Unyvero LRT/Pneumonia Control Panel M404 in demonstrating adequate system performance.
Expected results when controls are analyzed on the Unyvero System are listed in Table 1 for the Unyvero LRT Application and Table 2 for the Unyvero HPN Application.
2 Reviews
-
Original
Unyvero LRT/Pneumonia Control Panel M404 is intended for use as an external positive and negative quality control to monitor the performance of in vitro laboratory nucleic acid testing procedures for the qualitative detection of bacterial targets. The originality of a company is shown by its product. And the products on Gentaur are highly recommendable and original
-
Unyvero LRT/Pneumonia Control Panel M404
Unyvero LRT/Pneumonia Control Panel M404 is intended for use as an external positive and negative quality control to monitor the performance of in vitro laboratory nucleic acid testing procedures for the qualitative detection of bacterial targets; Acinetobacter spp., Chlamydia pneumoniae, Citrobacter freundii, Enterobacter cloacae complex, Enterobacter aerogenes†, Escherichia coli, Haemophilus influenzae, Klebsiella oxytoca, Klebsiella pneumoniae, Klebsiella variicola, Legionella pneumophila, Moraxella catarrhalis, Morganella morganii, Mycoplasma pneumoniae, Pneumocystis jirovecii†, Proteus spp., Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus, Stenotrophomonas maltophilia, Streptococcus pneumoniae, and antibiotic resistance markers; ctx-M, imp†, kpc, mecA, mecC†, ermB†, ndm, oxa-23, oxa-24, oxa-48, oxa-58, vim, tem, sul1†, shv†, gyrA83/87† (E.coli and P.aeruginosa) by the Unyvero Lower Respiratory Tract (LRT) Application and Unyvero Hospitalized Pneumonia (HPN) Application performed on the Unyvero System. Unyvero LRT/Pneumonia Control Panel M404 is composed of synthetic DNA specifically designed for and intended to be used with the Unyvero LRT and Unyvero HPN Applications.