Description
MMQCI Unyvero LRT Verification Panel M408 Part Number: M408
Kit Contains: 27 tubes x 180µL
4 each of Verification 1, Verification 2, Verification 3,
Verification 4, Verification 5; 7 of Verification NEG
Unyvero LRT Verification Panel M408 DataSheet
INTENDED USE:
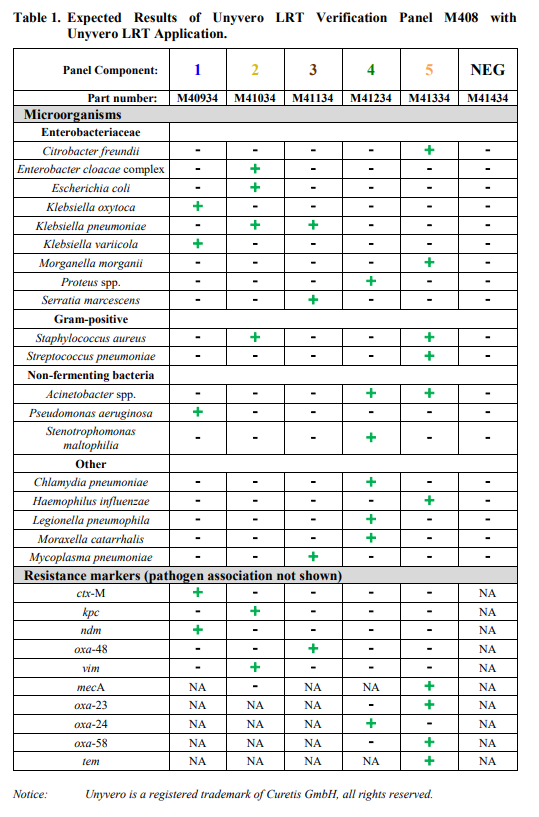
Unyvero LRT Verification Panel M408 is intended for use as external reference material to monitor the performance of in vitro laboratory nucleic acid testing procedures for the qualitative detection of bacterial targets and antibiotic resistance markers by the Unyvero Lower Respiratory Tract (LRT) Application performed on the Unyvero System. Unyvero LRT Verification Panel M408 is composed of synthetic DNA specifically designed for use with the Unyvero LRT Application. The bacterial targets and antibiotic resistance markers detected and identified by the Unyvero LRT Application are listed in Table 1.
PRODUCT SUMMARY and PRINCIPLE:
Unyvero LRT Verification Panel M408 is composed of 6 panel components; Unyvero LRT Verification 1, Unyvero LRT Verification 2, Unyvero LRT Verification 3, Unyvero LRT Verification 4, Unyvero LRT Verification 5, and Unyvero LRT Verification NEG. The LRT Verification components 1-5 contain surrogate control material composed of synthetic DNA corresponding to genome segments of the pathogens and associated antibiotic resistance markers listed in Table 1. The sequence of the synthetic DNA has been confirmed by bi-directional Sanger sequencing. Unyvero LRT Verification NEG contains no DNA.
Well-characterized reference materials can be useful for routine monitoring, verification and validation of new assays, training and troubleshooting.1, 2
COMPOSITION:
The Unyvero LRT Verification Panel M408 is comprised of 27 tubes, 4 tubes of each Verification 1, 2, 3, 4 and 5, and 7 tubes of Verification NEG, 180µL each. Unyvero LRT Verification 1, 2, 3, 4 and 5 contain synthetic DNA suspended in a non- infectious solution of buffers, preservatives and stabilizers. Unyvero LRT Verification NEG contains buffers and preservatives. Table 1 lists the pathogens and antibiotic resistance markers that are monitored by the Unyvero LRT Verification Panel M408 when tested with the Unyvero LRT Application on the Unyvero System.
PRECAUTIONS, WARNINGS and LIMITATIONS:
- Do not dilute or transfer to another storage
- This product is intended for in vitro analytical testing and is provided for Research Use Only (RUO).
- This product is for use with Unyvero LRT Application on the Unyvero It does not contain the entire genome of pathogens listed in Table 1.
- This product does not contain any biological material of human or animal origin. Universal Precautions are NOT required when handling this
- Quality control materials should be used in accordance with local, state, federal regulations and accreditation
- Unyvero LRT Verification Panel M408 cannot be cloned, sold, or transferred without the explicit written consent of
INSTRUCTIONS FOR USE:
- Allow the panel component to be tested to come completely to room temperature (18° – 25°C).
- Use Unyvero LRT Verification Panel M408 as Do not dilute or transfer to other tubes.
- Follow procedures listed in the Unyvero Instructions for Use provided by
- Immediately before use, mix the control thoroughly by pulse-vortexing for 5-10 Gently tap the tube several times on the bench to remove any liquid caught in the cap before opening the tube.
- Remove the Transport Cap by gently pulling it upward and dispose of it. If necessary, use a new pipette tip to remove any bubbles on the surface of the Unyvero Sample
- Transfer 180µL of the vortexed control into the Sample Tube. Close it with the Unyvero Sample Tube Cap provided in the kit by aligning the small nodules on the neck of the Sample Tube with the openings on the Press down to lock in place.
- Continue to process according to the Unyvero Instructions for
References
- Validation and Verification of Multiplex Nucleic Acid Assays. 2nd ed. CLSI guideline MM17. Wayne, PA: Clinical and Laboratory Standards Institute; 2018.
- Establishing Molecular Testing in Clinical Environments. 1st ed. CLSI guideline MM19. Wayne, PA: Clinical and Laboratory Standards Institute; 2011.
STORAGE and STABILITY:
The Unyvero LRT Verification Panel M408 should be stored refrigerated at 2-8°C. Unopened Unyvero LRT Verification Panel M408 material is stable through the expiration date printed on the kit label when consistently stored refrigerated. Unyvero LRT Verification Panel M408 components are for single use. Discard after use according to your local and federal regulations.
EXPECTED VALUES:
The laboratory should follow Good Laboratory Practice (GLP) and establish its own performance characteristics for Unyvero LRT Verification Panel M408 in demonstrating adequate system performance.
Expected results when Unyvero LRT Verification Panel M408 is analyzed with the Unyvero LRT Application on the Unyvero System are listed in Table 1.
3 Reviews
-
Lovely
my overall interaction with Gentur staff and its products is lovely. Feeling very happy
-
its good
The company is good in its service with well-mannered workers
-
Unyvero LRT Verification Panel M408
Unyvero LRT Verification Panel M408 is intended for use as external reference material to monitor the performance of in vitro laboratory nucleic acid testing procedures for the qualitative detection of bacterial targets and antibiotic resistance markersby the Unyvero Lower Respiratory Tract (LRT) Application performed on the Unyvero System. Unyvero LRT Verification Panel M408 is composed of synthetic DNA specifically designed for use with the Unyvero LRT Application.










