Description
MMQCI Xpert BCR-ABL 0%IS
Part Number: C13112-5
Kit Contains: 5 bottles x 4mL
INTENDED USE:
The Xpert BCR-ABL 0%IS is intended for use as an assayed external quality control to monitor the performance of the in vitro quantitative detection of BCR- ABL1 translocation mRNA e14a2/b3a2 transcripts and the ABL1 endogenous control mRNA transcript when analyzed using the Xpert® BCR-ABL Ultra assay on Cepheid GeneXpert® Instrument Systems.
The Philadelphia chromosome, a translocation between the ABL1 gene on chromosome 9 and the BCR gene on chromosome 22, designated as t(9;22), generates the fusion gene BCR-ABL1 which is present in most chronic myelogenous leukemia patients. Quantitative monitoring of BCR-ABL1 transcripts in patient blood is an important tool for measuring response to therapy. In 2009, the World Health Organization (WHO) developed a panel of four BCR- ABL1 primary standards to establish an international scale (IS), a standardized method for reporting assay results as a ratio of fusion transcripts to control gene transcripts (%IS), useful to the harmonization of patient care across laboratories worldwide.1,2 The %IS can also be expressed as molecular response (MR), the log reduction from a standardized baseline of 100% on the IS. The Xpert BCR-ABL IS Panel C130 is traceable to the WHO International Genetic Reference Panel for Quantitation of BCR-ABL Translocation (WHO Reference Panel), NIBSC code 09/138, and designed for use with the Xpert BCR-ABL Ultra assay which reports on the international scale.
PRODUCT SUMMARY and PRINCIPLE:
Xpert BCR-ABL 0%IS is composed of one level, Xpert BCR-ABL 0%IS, which contains synthetic RNA transcripts of control gene ABL1. This control is designed to represent a sample with no fusion gene BCR-ABL1 present when analyzed on the GeneXpert system with the Xpert BCR-ABL Ultra assay. Xpert BCR-ABL 0%IS is a component of Xpert BCR-ABL IS Panel C130, part number C130.
Validation and Value Assignment
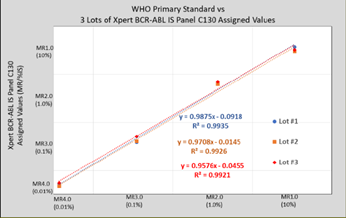
MMQCI manufactured 3 lots of Xpert BCR-ABL IS Panel C130 and tested them alongside the WHO Reference Panel, using one cartridge lot of the Xpert BCR- ABL Ultra assay. Grubb’s outlier and the Bland-Altman tests were applied, lot- specific Correction Factors (CF) were calculated, and WHO-traceable %IS/MR values were assigned to each level of Xpert BCR-ABL IS Panel C130 for all 3 lots according to NIBSC Instructions for Use3. Figure 1. compares the 3 lots of Xpert BCR-ABL IS Panel C130 to the 4 members of the WHO Reference Panel. New lots of Xpert BCR-ABL IS Panel C130 will be assigned lot-specific %IS/MR values in the same manner.
Figure 1. Three lots of Xpert BCR-ABL IS Panel C130 calibrated to the WHO International Genetic Reference Panel for Quantitation of BCR-ABL Translocation.
COMPOSITION:
Xpert BCR-ABL 0%IS contains 5 single use bottles of Xpert BCF-ABL 0%IS consisting of synthetic ABL1 control gene transcipt suspended in a stabilizing matrix with a non-infectious solution of buffers and preservatives.
STORAGE and STABILITY:
Xpert BCR-ABL 0%IS should be stored at -20oC. Unopened Xpert BCR-ABL 0%IS material is stable through the expiration date printed on the kit label when consistently stored frozen. Xpert BCR-ABL 0%IS is for single use. Discard after use according to your local and federal regulations.
INSTRUCTION FOR USE:
- Allow the Xpert BCR-ABL 0%IS component to be tested to come completely to room temperature (18oC-25oC), approximately 30 minutes, until bottles are warm to the
- Immediately before pipetting, thoroughly mix the C130 panel component by inverting 8 times followed by 2 pulse vortexes, 2-3 seconds each at maximum
- Add 4mL of the Xpert BCR-ABL 0%IS component to 100µL of Proteinase K in a conical tube, as you would a blood specimen.
- Continue with the assay procedure according to manufacturer’s
- Discard after use according to local and federal
PRECAUTIONS and WARNINGS:
- Use the control as Do not dilute or transfer to another storage tube.
- This product is intended for in vitro diagnostic use
- Use Xpert BCR-ABL 0%IS only with Xpert BCR-ABL Ultra
- Xpert BCR-ABL 0%IS is not intended to be used for calibration of the Xpert® BCR-ABL Ultra
- This product is slightly cloudy in
- This product does not contain any biological material of human or animal Universal Precautions are NOT required when handling this product.
- Xpert BCR-ABL 0%IS cannot be cloned, sold, or transferred without the explicit written consent of
EXPECTED VALUES:
The expected result of Xpert BCR-ABL 0%IS when tested with Xpert BCR-ABL Ultra assay on the GeneXpert system is: Negative (Sufficient ABL1 transcript).
|
Control |
Reported Result |
|
Xpert BCR-ABL 0%IS |
Negative (Sufficient ABL transcript) |
Routine use of quality controls that are consistent lot-to-lot assists the laboratory in identifying shifts, trends, and increased frequency of random errors caused by variations in the test system.
References
1Branford S et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood 2008, 112:3330-38
2White HE et al. Establishment of the first World Health Organization International Genetic Reference Panel for quantitation of BCR-ABL mRNA. Blood 2010, 116:e111-117
31st WHO International Genetic Reference Panel for Quantitation of BCR-ABL Translocation, NIBSC code: 09/138 Instructions for use (Version 4.0, Dated 13/12/2012)
3 Reviews
-
Schnell geliefert, innerhalb von 4 Werktagen.
GeneXpert BCR-ABL Qualitätskontrollen mit Materialnummer 0868-C130
-
Quick shipping
Gentaur always fulfils its promise. They always shipped their products on time without any delay. Highly recommended!
-
Xpert BCR-ABL 0%IS
The Xpert BCR-ABL 0%IS is intended for use as an assayed external quality control to monitor the performance of the in vitro quantitative detection of BCRABL1 translocation mRNA e14a2/b3a2 transcripts and the ABL1 endogenous control mRNA transcript when analyzed using the Xpert® BCR-ABL Ultra assay on Cepheid GeneXpert® Instrument Systems.