Description
The SD Malaria Control Kit contains a Positive Control (PC), which is precoated with recombinant histidine-rich protein II (HRP-II) antigen and recombinant lactate dehydrogenase(LDH) antigen from Malaria Plasmodium vivax and a Negative Control (NC). Concentrations of the PC equivalent to 200 parasites per microliter of blood.
The SD Malaria Positive and Negative controls are prepared to use as a consumer’s internal control to check SD BIOLINE Malaria Ag P.f, SD BIOLINE Malaria Ag P.f/Pan and SD BIOLINE Malaria Ag P.f/P.v test kit. The positive and negative controls are used only with related test kits.
SD Malaria Positive and Negative Controls DataSheet
Materials provided and active ingredients of main component
1. The SD Malaria Control Kit contains the following items to perform the assay:
• 1 Positive control swab
• 1 Negative control swab
• 2 Disposable droppers
• 2 Specimen collection tubes containing extract buffer (0.5ml)
• 1 Instructions for use
2. Active ingredients of main component
• PC includes; Recombinant HRP-II (2.0 ± 0.4ng), Recombinant P.v LDH (5 ± 1μg)
• Extract buffer includes; Bovine serum albumin (q.s.), Phosphate Saline Buffer (q.s.), Triton X-100 (q.s.), Sodium azide (q.s.)
Kit storage and stability
1. The SD Malaria Control Kit should be stored at a temperature between 1 ˚C and 30 ˚C. Do not freeze the components.
2. Check the humidity indicator on the desiccant for color change and throw the pouch if the color indicates saturation
(OK if yellow. Discard if green - do not use control swabs, discard.).
3. Do not use the components beyond the expiration date. The shelf life of the kit is as indicated on the pouch and outer package.
4. Do not use the kit components if the pouch is damaged or the seal is broken.
Warnings
1. 3 Test devices can be tested with extract buffer after dissolving a control swab.
2. The instructions must be followed exactly to achieve accurate results. Any individual performing an assay with this product must be trained in its use and must be experienced in laboratory procedures.
3. Do not remove the control swabs from their individual pouch before their use.
4. After dissolved, extract buffer should be tested immediately. If extract buffer is not tested immediately, it recommended to be used until 6 hours when refrigerated at a temperature between 2 °C and 8 °C.
5. Wear protective gloves while handling control swabs and wash hands thoroughly afterwards.
6. Do not reuse the control swabs.
7. Do not try to dilute the Malaria controls with extracted buffer.
8. Do not use the controls with different brand's contents.
9. Decontaminate and dispose of all specimens, reaction kits and potentially contaminated materials (i.e. control swab, sample applicator) in a biohazard container as if they were infectious waste
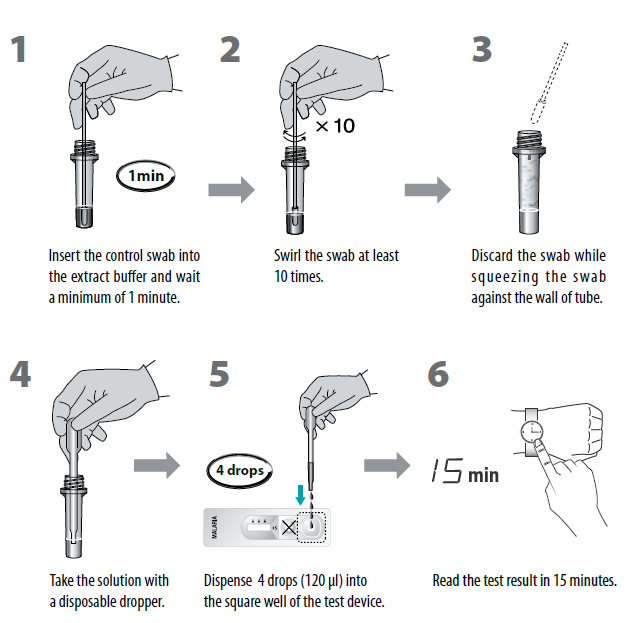
Test Procedure (Refer to figure)
1. Allow all kit components to come to 15 - 30 ˚C prior to testing.
2. Remove the positive or negative control swabs from the pouch.
3. Insert the end of the control swab into the specimen collection tube containing extract buffer and wait a minimum of 1 minute.
4. Swirl the swab at least 10 times until the control has been dissolved into the extract buffer and discard the swab.
5. Remove the test device from the foil pouch and place it on a flat, dry surface.
6. Using a disposable dropper, dispense 4 drops (120 μl) of the solution into the square shaped extract buffer well of test device.
7. Read the test result in 15 minutes.
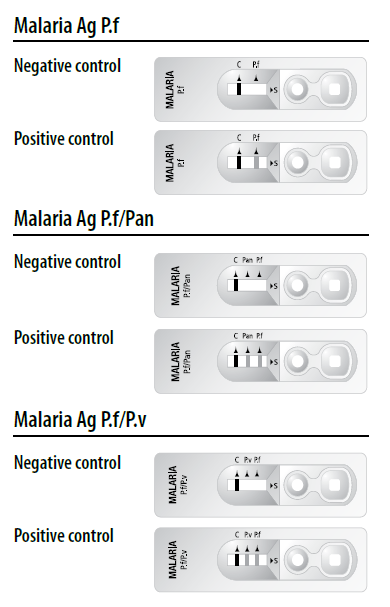
Test interpretation (Refer to figure)
• A colored control line will appear in the left section of the result window to show that the test is working properly.
• The right section of the result window indicates the test results. If another colored line(s) appear in the right section of the result window, this is(these are) the test line(s).
1. Positive control : Two or three lines appear.
The presence of control line (C) and test line(s) within the result window, regardless of which line appears first, indicates a positive result.
*Caution : The presence of any line, no matter how faint, the result is considered positive.
2. Negative control : Only one line (C) appears.
The presence of only the control line (C) within the result window indicates a negative result.
Note: If the test result of the control swab is not as expected, repeat test with a new test device and extracted control
solution. If any problems arise that cannot be resolved by this, please contact us (info@gentaur.com).
1 Review
-
obbligato
il modo in cui gestiscono la loro gestione e spedizione con qualità è molto apprezzabile