Description
The ZeptoMetrix® Mixed Titer Bacteria Panel for Platelets* is a 12 member panel containing the most common bacterial contaminants in stored human platelets.
Designed to assist in the validation of clinical assays and for technician training and proficiency
assessment, the ZeptoMetrix Mixed Titer Bacteria Panel for Platelets has demonstrated performance on commercially available tests* for bacterial contamination in human platelet concentrates.
Each Mixed Titer Bacteria Panel for Platelets consists of 12 x 0.1 ml vials, representing a total of 10 positive and 2 negative samples. A collection of 8 different bacteria - four Gram-positive and four Gram-negative -ensures a broad assessment of those potential contaminants commonly tested for during platelet processing.
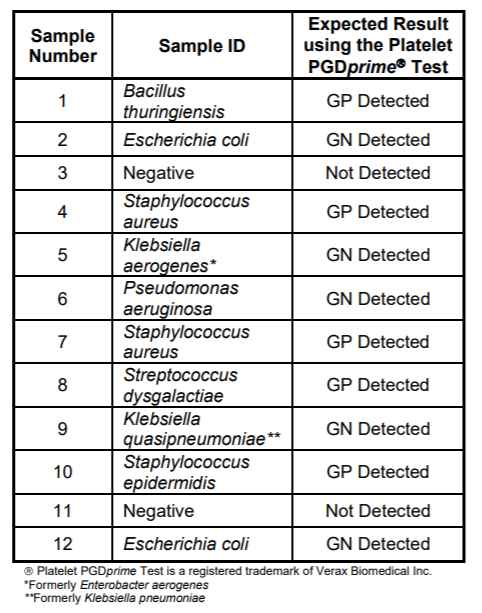
The mixed titer panel contains vials in the following configuration:
1. Bacillus thuringiensis
2. Escherichia coli - medium titer
3. Negative
4. Staphylococcus aureus - medium titer
5. Klebsiella aerogenes
6. Pseudomonas aeruginosa
7. Staphylococcus aureus - high titer
8. Streptococcus dysgalactiae
9. Klebsiella quasipneumoniae
10. Staphylococcus epidermidis
11. Negative
12. Escherichia coli - high titer
*The ZeptoMetrix Mixed Titer Bacteria Panel for Platelets has demonstrated performance with the Verax Biomedical Platelet PGD® Test and Platelet PGDprime® Test.
This product is for Research Use Only and is not for use in diagnostic procedures.
Zeptometrix | 0820000 | Mixed Titer Bacteria Panel for Platelets DataSheet
PRODUCT DESCRIPTION:
The Mixed Titer Bacteria Panel for Platelets contains 12 x 0.1 mL microbial samples. This panel is designed for testing the performance of laboratory personnel using a post-storage bacterial detection test for platelets. Some panel members are negative and should not produce a positive signal. Positive samples should yield a wide range of signal strengths.
BIOSAFETY:
This panel contains live Biosafety Level 2 microorganisms and must be used within Biological Safety Level 2 facility or cabinet. Please consult your institution’s regulations regarding the use of this panel. For a detailed discussion on biological safety see the 5th edition of Biosafety in Microbiological and Biomedical Laboratories (BMBL), published by the CDC at http://www.cdc.gov/biosafety/publications/bmbl5/in dex.htm.
PRECAUTIONS:
• Use Universal Precautions, this panel contains live bacteria.
• To avoid cross-contamination, use separate pipette tips for all reagents.
RECOMMENDED STORAGE AND HANDLING: Mixed Titer Bacteria Panels for Platelets should be stored ≤-65ºC to maximize the shelf-life. The panels can be stored at –20ºC for one month with minimal loss of activity. Do not use a frost-free freezer. Do not thaw and refreeze the samples. Samples should be run within 30 minutes of thawing.
INSTRUCTIONS FOR THE PLATELETS:
• Platelets used for testing and reconstitution should be transfusion quality, 2-8 days post-collection and stored properly (22ºC, rocking). The platelets should show no signs of deterioration or coagulation. The pH should be above 6.2.
• Following the manufacturer’s instructions test the platelet sample to ensure that it is non-reactive prior to using the platelets to reconstitute the panel members.
INSTRUCTIONS FOR USE:
1. Samples to be tested should be thawed at room temperature for 15 minutes prior to use.
2. Ensure that the sample is in the bottom of the tube before adding the platelets.
3. Reconstitute the samples with 1 ml of platelets. Mix by gentle inversion. Do Not Vortex.
4. Perform the bacterial detection test per the instructions of the manufacturer, including the volume of sample to use and the number of samples to test in a single session.
DO NOT USE IN HUMANS. FOR RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES.
These products are intended for research, product development or manufacturing use only. These products are NOT intended for use in the manufacture or processing of injectable products subject to licensure under section 351 of the Public Health Service Act or for any other product intended for administration to humans.