Description
The Axis-Shield Homocysteine Control Kit is intended to be used as an assayed quality control serum when used for the quantitative measurement of total L-homocysteine in human serum or plasma.
Axis-Shield Homocysteine Control Kit DataSheet
Summary and Principle
The use of quality control material is indicated as an objective assessment of the precision of methods and techniques in use and is an integral part of good laboratory practises. Three levels of control are available to allow performance monitoring within the clinical range.
Contents
3 vials (1.5 mL each) contain L-homocysteine in processed human serum in the following concentration ranges:
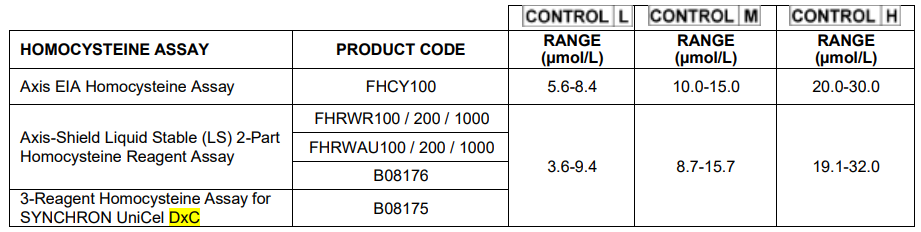
Beckman Coulter, SYNCHRON and UniCel are trademarks of Beckman Coulter, Inc. and are registered in the USPTO. All other trademarks are the property of their respective owners.
Precautions
For In Vitro Diagnostic Use.
Caution: Federal law restricts this device to sale by or on the order of a physician
The controls contain human sourced and potentially infectious components. Components sourced from human blood have been tested and found to be non-reactive for Hepatitis B Surface Antigen (HBsAg), HIV-1 Antigen (HIVAg) or HIV-1 RNA, HCV antibody,HIV- 1/2 antibody, HTLV-1/2 antibody and Hepatitis B core Antibody (HBc) No known test method can offer complete assurance that products derived from human sources will not transmit infection. Therefore all human sourced materials should be considered potentially infectious. It is recommended that these materials be handled in accordance with Biosafety Level 21 or local/national guidelines on laboratory safety procedures. Controls contain <0.10% sodium azide as preservative.
Storage and Stability
The Axis-Shield Homocysteine Control Kit must be stored refrigerated (2-8ºC). The controls are stable until the expiration date when stored and handled as directed. Do not use past expiration date.
Procedure and Handling
Each control should be treated as a patient sample and run in accordance with the instructions accompanying the instrument, kit or reagent being used.
Before sampling, allow the control to reach room temperature (18-25ºC) and swirl gently before use to ensure homogeneity. Return the control to storage at 2-8ºC immediately after use.
Dispose of any discarded materials in accordance with local waste management regulations.
Assignment of values
Individual laboratory means should fall within the corresponding acceptable range. Variations over time and between laboratories may be caused by differences in laboratory technique, instrumentation and reagents, or by test method modifications. It is recommended that each laboratory establishes its own mean and acceptable ranges and use those provided only as a guide.
Limitations
This product must not be used past the expiration date.
If there is evidence of microbial contamination or excessive turbidity in the product discard the vial. This product is not intended for use as a calibrator.
Technical Support
For Technical Support please contact your local distributor or representative.
1 Review
-
higher quality
I ordered products from gentaur of high quality with 100% satisfaction