Type I Collagen N-Telopeptide (NTX-I) Detection Kit
- SKU:
- 445-6040
- Size:
- 1 kit
- Shipping:
- Gel Packs
- Storage:
- -20 C
Description
Type I Collagen N-Telopeptide (NTX-I) Detection Kit - Cat Number: 6040 From Chondrex.
Research Field: Arthritis, ECM
Clonality: N/A
Cross-Reactivity:
Host Origin: N/A
Applications: N/A
Isotype: N/A
Detection Range: 20 ng/ml-0.31 ng/ml
Sample Type: Serum, Plasma, Urine
Concentration: N/A
Immunogen:
PRODUCT SPECIFICATIONS
DESCRIPTION: ELISA kit to quantify NTX-I fragments/peptides
FORMAT: Pre-coated 96-well ELISA Plate with removeable strips
ASSAY TYPE: Sandwich ELISA
ASSAY TIME: 4 hours
STANDARD RANGE: 20 ng/ml to 0.31 ng/ml
NUMBER OF SAMPLES: Up to 40 (duplicate) samples/plate
SAMPLE TYPES: Urine, Serum, and Plasma
RECOMMENDED SAMPLE DILUTIONS: 1:1 (at least)
CHROMOGEN: TMB (read at 450 nm)
STORAGE: -20°C
VALIDATION DATA: Intra-Assay (0.8-7.4%)/Inter-Assay (4.8-6.9%)/Spiking Test (92-102%)
NOTES:
INTRODUCTION
Collagen is the most abundant protein in the mammalian body and lends structural integrity to tissues as the primary component of the extracellular matrix (1). Among the many types of collagen, Type I collagen is the main component of bone, tendon, skin, and other tissues (2). In fact, type I collagen makes up 20% of bones by mass, accounting for more than 90% of the organic components. As a result, degraded type I collagen peptides appear in serum and urine in early stages of bone loss or metabolism, and indicate bone turnover levels (3, 4). Proteinases mediate resorption of type I collagen from bone and generate specific peptides of degraded collagen. For example, matrix metalloproteinases (MMPs) exclusively produce C-terminal degraded peptides, ICTP, from type I collagen, while cathepsin K produces CTXI peptides from the C-terminus and NTX-I peptides from the N-terminus of type I collagen (3). Patients with osteoporosis display reduced bone mass, indicating that bone cell destruction is greater than bone cell production. In fact, bone loss is increased by lower levels of estrogen after menopause. During the progression of osteoporosis, ICTP and NTX-I are observed in serum as well as urine, and are useful as markers of osteoporosis (4-6). Additionally, bone metastasized cancers, such as breast cancer, prostate carcinoma, and lung cancer, show increased NTX-I levels in serum and urine due to high levels of degraded type I collagen (7, 8). Thus, degraded peptides of type I collagen are useful tools for evaluating bone metabolism levels in many diseases not only in human patients, but also in mouse disease models of cancer, osteoporosis, osteoarthritis, and rheumatoid arthritis. Chondrex, Inc. provides a mouse NTX-I Detection ELISA Kit (Cat # 6040) as well as a CTX-I Detection ELISA Kit (Cat # 6033) and a Creatinine Assay Kit (Cat # 6041) which is useful for normalizing results among urine samples. Please contact support@chondrex.com or visit www.chondrex.com for more information.
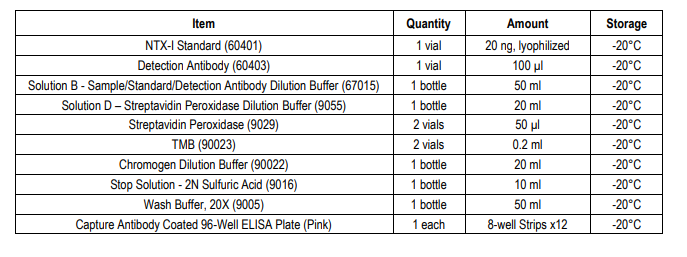
KIT COMPONENTS
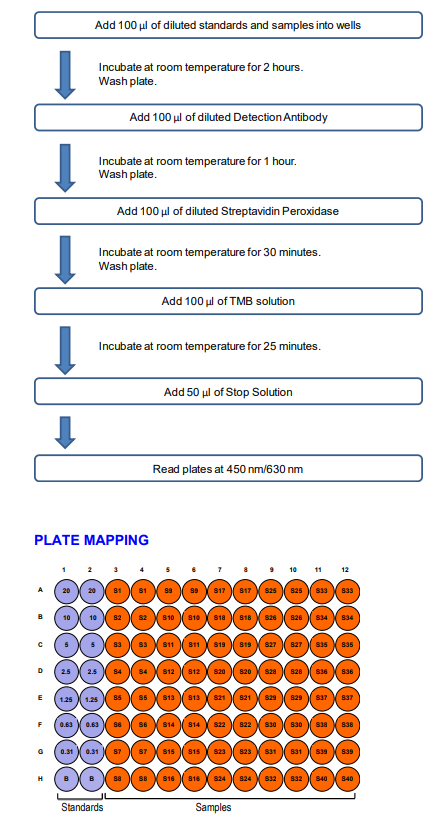
ASSAY OUTLINE
NOTES BEFORE USING ASSAY
NOTE 1: It is recommended that the standard and samples be run in duplicate.
NOTE 2: Warm up all buffers to room temperature before use.
NOTE 3: Crystals may form in Wash Buffer, 20X when stored at cold temperatures. If crystals have formed, warm the wash buffer by placing
the bottle in warm water until crystals are completely dissolved.
NOTE 4: Measure exact volume of buffers using a serological pipet, as extra buffer is provided.
NOTE 5: Cover the plate with plastic wrap or a plate sealer after each step to prevent evaporation from the outside wells of the plate.
NOTE 6: For partial reagent use, please see the assay protocol’s corresponding step for the appropriate dilution ratio. For example, if the protocol dilutes 50 µl of a stock solution in 10 ml of buffer for 12 strips, then for 6 strips, dilute 25 µl of the stock solution in 5 ml of buffer. Partially used stock reagents may be kept in their original vials and stored at -20⁰C for use in a future assay.
NOTE 7: This kit contains animal components from non-infectious animals and should be treated as potential biohazards in use and for disposal.
ASSAY PROCEDURE
1. Prepare Standard Dilutions: The recommended standard range is 0.31 - 20 ng/ml. Dissolve one vial of NTX-I standard in 1 ml of Sample/Standard/Detection Antibody Dilution Buffer (Solution B) for the 20 ng/ml standard. Then serially dilute it with Solution B. For example, mix 250 µl of the standard (20 ng/ml) with an equal volume of Solution B to make a 10 ng/ml solution, and then repeat it five more times for 5, 2.5, 1.25, 0.63, and 0.31 ng/ml solutions. The remaining 20 ng/ml standard stock may be stored at -20°C for use in a second assay. Chondrex, Inc. recommends making fresh serial dilutions for each assay.
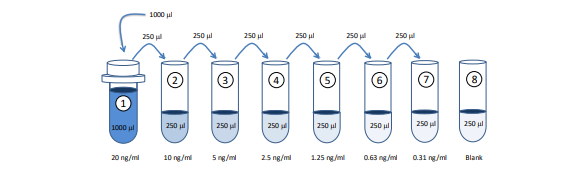
Prepare Sample Dilutions: Centrifuge samples at 10,000 rpm at 4°C for 3 minutes to remove insoluble materials and lipids. Dilute the supernatants with an equal volume of Solution B. For example, take 100 µl of supernatant, and mix with 100 µl of Solution B. If the OD values of samples are higher than the OD values of the 20 ng/ml standard, re-assay the sample at a higher dilution.
NOTE: Dilute samples at least 1:1 with Solution B depending on the estimated NTX-I level in the samples. Two to three different sample dilutions are recommended if the NTX-I levels in the samples are unknown.
3. Add Standards and Samples: Add 100 µl of Solution B (blank), standards, and samples to designated wells. Incubate at room temperature for 2 hours.
4. Dilute Wash Buffer: Dilute 50 ml of Wash Buffer, 20X in 950 ml of distilled water (1X wash buffer). Wash the plate with 1X wash buffer at least 3 times using a wash bottle with manifold or an automated plate washer. Empty the plate by inverting it and blotting on a paper towel to remove excess liquid. Do not allow the plate to dry out.
Add Detection Antibody Solution: Prepare detection antibody solution with Solution B as shown in the following table. Add 100 µl of detection antibody solution to each well and incubate at room temperature for 1 hour.

6. Wash: Wash the plate with 1X wash buffer at least 3 times using a wash bottle with manifold or an automated plate washer. Empty the plate by inverting it and blotting on a paper towel to remove excess liquid. Do not allow the plate to dry out.
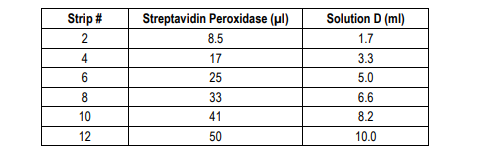
7. Add Streptavidin Peroxidase Solution: Prepare streptavidin peroxidase solution with Streptavidin Peroxidase Dilution Buffer (Solution D) as shown in the following table. Add 100 µl of streptavidin peroxidase solution to each well and incubate at room temperature for 30 minutes.
10. Stop: Stop the reaction with 50 µl of 2N Sulfuric Acid (Stop Solution) to each well.
11. Read Plate: Read the OD values at 450 nm. If the OD values of samples are greater than the OD values of the highest standard, reassay the samples at a higher dilution. A 630 nm filter can be used as a reference.
CALCULATING RESULTS
1. Average the duplicate OD values for the blank, standards, and test samples.
2. Subtract the “blank” (B) values from the averaged OD values in step 1.
3. Plot the OD values of standards against the concentration of NTX-I (ng/ml). Using a log/log plot will linearize the data. Figure 1 shows a representative experiment where the standard range is 0.31 - 20 ng/ml.
4. The ng/ml of NTX-I in test samples can be calculated using regression analysis. Figure 1 - A Typical Standard Curve for the NTX-I Detection ELISA Kit.
REFERENCES
1. E. Hohenester, J. Engel, Domain structure and organisation in extracellular matrix proteins. Matrix Biol 21, 115-28 (2002).
2. K. Gelse, E. Pöschl, T. Aigner, Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev 55, 1531-46 (2003).
3. G. Wheater, M. Elshahaly, S. Tuck, H. Datta, L. van, The clinical utility of bone marker measurements in osteoporosis. J Transl Med 11, 201 (2013).
4. M. Bonde, C. Fledelius, P. Qvist, C. Christiansen, Coated-tube radioimmunoassay for C-telopeptides of type I collagen to assess bone resorption. Clin Chem 42, 1639-44 (1996).
5. K. Lee, M. Lee, C. Chung, W. Seong, S. Lee, M. Park, et al., Measurement of urinary N-telopeptides and serum C-telopeptides from type I collagen using a lateral flow-based immunoassay. Sensors (Basel) 13, 165-74 (2012).
6. J. Clemens, M. Herrick, F. Singer, D. Eyre, Evidence that serum NTx (collagen-type I N-telopeptides) can act as an immunochemical marker of bone resorption. Clin Chem 43, 2058-63 (1997).
7. M. Koizumi, E. Ogata, Bone metabolic markers as gauges of metastasis to bone: a review. Ann Nucl Med 16, 161-8 (2002).
8. K. Jung, M. Lein, Bone turnover markers in serum and urine as diagnostic, prognostic and monitoring biomarkers of bone metastasis. Biochim Biophys Acta 1846, 425-38 (2014).






