E7 Oncoprotein Rapid Test Kit | E7-FAMID
- SKU:
- E7-FAMID-GEN
- Availability:
- IN STOCK
- Size:
- 1 kit ( 96 Tests)
Description
E7 Oncoprotein Rapid Test Kit | E7-FAMID | FAMID Biomedical Technology
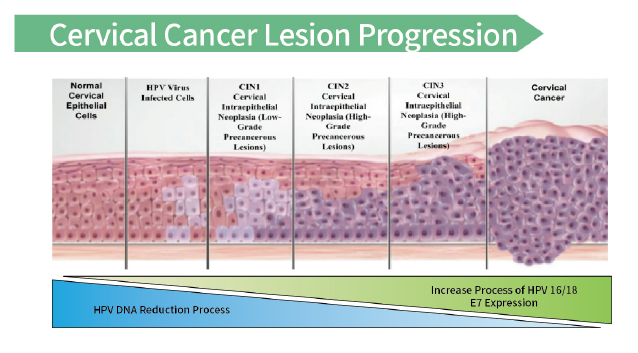
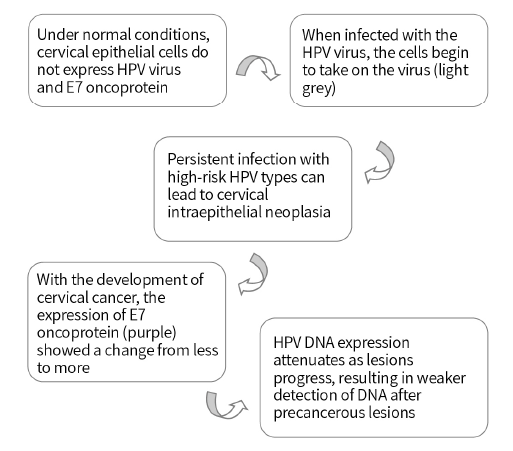
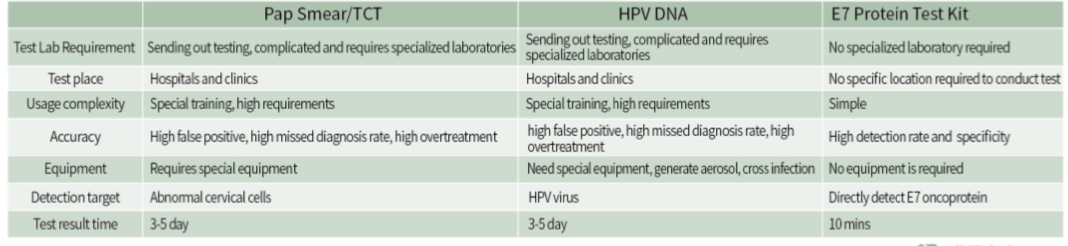
E7 protein - a marker for the progression of cervical lesions
|
Characteristics |
Description |
|
The detection elements are clear |
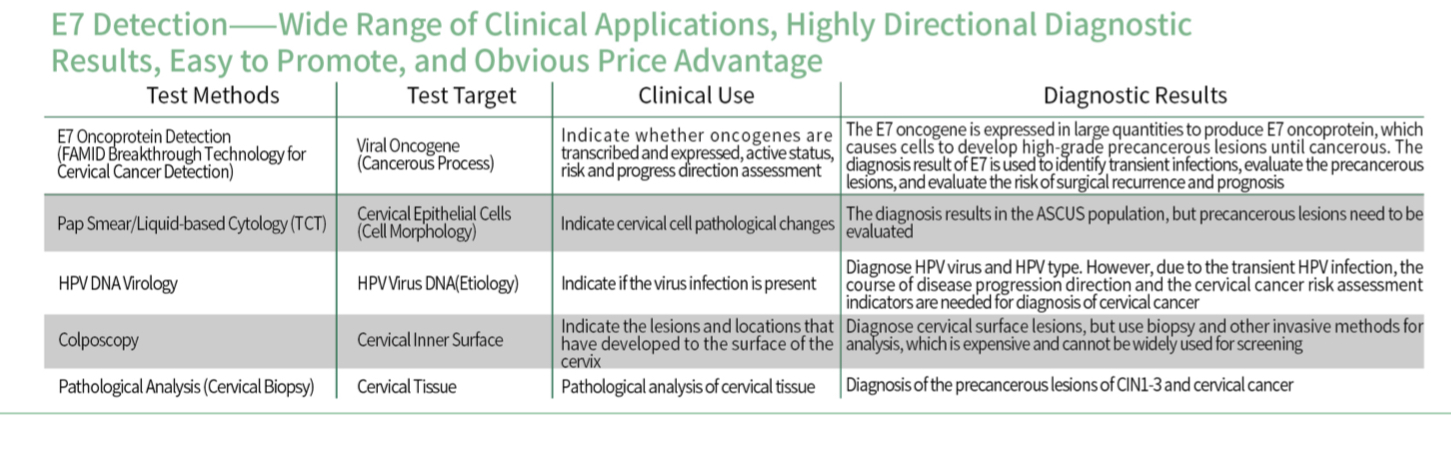
● This kit detects E7 protein- ● Expression of E7 protein in cervical epithelial cells ●cervical cancer lesions |
|
The detection index is highly indicative |
● E7 protein reflects patient lesions and is one of the main indicators for cervical cancer risk assessment ●Provide cervical cancer risk assessment indicators ●Provide a basis for subsequent cervical cancer treatment |
|
Detection helps in the diagnosis and precise treatment of cervical diseases |
● Effectively reduce over-diagnosis and panic of HPV transient infection ● It can directly determine the low-risk group of cervical cancer and prevent overtreatment or missed diagnosis |
|
The detection operation is convenient and the result is highly accurate
|
● Self-test at home using colloidal gold screening method ●Easy to operate, fast and accurate |
- People without TCT and DNA test results can directly use E7 protein detection kit (colloidal gold method) to detect E7 protein
- content People with only HPV DNA test results, if the DNA test result is positive, use E7 protein detection kit (colloidal gold method) to detect E7 protein
- For people with only CT test results, if the TCT test results are above ASC-US, use E7 protein detection kit (colloidal gold method) After surgery and postoperative recurrence, E7 protein is detected using E7 protein detection kit (colloidal gold method). Postoperative tissue margin section, plus E7 to evaluate the possibility of recurrence
- For people with negative TCT and HPV DNA, use E7 protein detection kit (colloidal gold method) to detect E7 protein. After cervical precancerous lesions, the weakened DNA expression leads to false negatives and missed diagnoses. The expression of E7 is relatively increased, and the risk of cancer is also higher. Active treatment is recommended.

1 Review
-
Fast and Supportive
I really like this cervical cancer screening test! It gives results quickly. Using the test was simple. The technical support, from the company was top notch whenever I needed help.






