Description
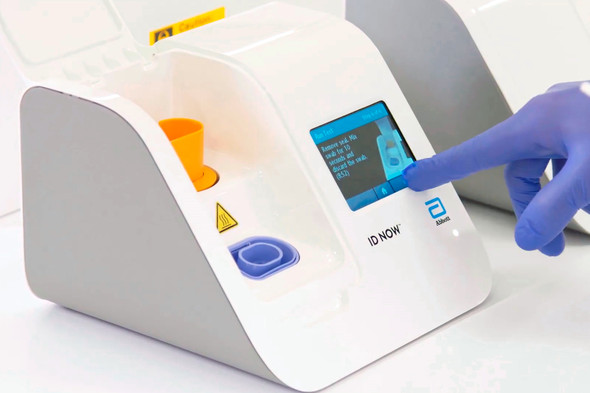
Abbott™ ID NOW™ COVID-19 Assay Kit High-quality molecular technology targets the COVID-19 RdRp gene for a positive result within 5 minutes, and a negative result within 13 minutes.
Description
- Leading molecular point-of-care (POC) platform in the United States
- Trusted by hospitals, physician offices, and urgent care facilities nationwide
- Emergency Use Authorization (EUA) supports flexible near-patient testing environments
- Sample types tested direct or in viral transport media (VTM) include throat swabs, nasal swabs, and nasopharyngeal swabs
- Room temperature storage eliminates the need for refrigeration
- Assay kit includes 24 tests, sample collection swabs, pipettes, and control swabs
- Control kit is available for addition positive and negative swabs
Specifications
| Nasal Swabs, Nasopharyngeal Swabs, and Throat Swabs | |
| CLIA | |
| Waived, Moderate, High | |
| 5 Minutes (Positive), 13 Minutes (Negative) | |
| None | |
| Point of Care COVID Testing | |
| RdRp | |
| Plastic | |
| Virus | |
| 6 Months from Date of Manufacture | |
| Ambient | |
| Yes | |
| Yes | |
| Cardiolipin IgA II Test |
| Kit | |
| 12 Positive and 12 Negative (Additional Controls Sold Separately) | |
| Yes | |
| Virus | |
| Molecular COVID Assay | |
| ID NOW™ Instrument | |
| 24 Tests | |
| 24/Kit | |
| See Package Insert | |
| See Package Insert | |
| Isothermal Molecular | |
| COVID-19 | |
| 24 Tests, Sample Collection Swabs, Pipettes, and Control Swabs | |
| 96 Tests |
1 Review
-
obbligato
il modo in cui gestiscono la loro gestione e spedizione con qualità è molto apprezzabile