Description
AZF System Y-chromosome | 01200-5 from Sacace Biotechnologies is available for delivery
Real Time PCR test for detection of the microdeletions in AZF regions: AZFa (sY84, sY86), AZFb (sY127, sY134), AZFc (sY254, sY255) of the human Y chromosome.
INTENDED USE: Diagnosis of a microdeletion of the Y chromosome permits the cause of the patients azoospermia/oligozoospermia to be established and to formulate a prognosis. The world literature, now based on several thousands of patients screened, indicates that, as a rule, clinically relevant deletions are found in patients with azoospermia or severe oligozoospermia with sperm concentrations <2 x 106/mL
Storage : AZF System Y-chromosome kit must be stored at -20°C. The kits can be shipped at 2-8°C and stored as indicated immediately on receipt
AZF System Y-chromosome (CE) 01200-5 DataSheet
INTRODUCTION
Y-chromosomal microdeletions are the second most frequent genetic cause of male infertility after the Klinefelter syndrome. In the last decade, many investigators have described the occurrence of microdeletions in infertile patients around the world and the molecular diagnosis of deletions has become an important test in the diagnostic workup of male infertility. Microdeletions occur in about one in 4000 men in the general population but its frequency is significantly increased among infertile men.
Azoospermic men have a higher incidence of microdeletions than oligozoospermic men and consequently deletion frequency found in different laboratories may vary from 2 to 10% (or even higher, see Fig. below) reflecting the composition of the study population.
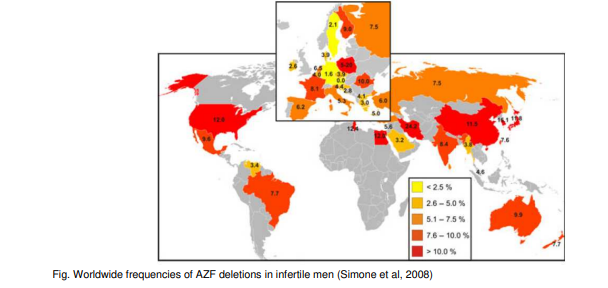
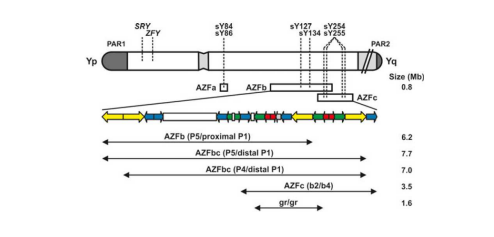
The complete physical map and sequence of MSY (Male-Specific Region Of The Y Chromosome) has been available since 2003. According to the present knowledge, the following recurrent microdeletions of the Y chromosome are clinically relevant and are found in men with severe oligo- or azoospermia (see fig. below): AZFa, AZFb (P5/proximal P1), AZFbc (P5/distal P1 or P4/distal P1), AZFc (b2/b4). The most frequent deletion type is the AZFc region deletion (~80%) followed by AZFa (0.5–4%), AZFb (1–5%) and AZFbc (1–3%) deletion. Deletions which are detected as AZFabc are most likely related to abnormal karyotype such as 46,XX male or iso(Y).
PRINCIPLE OF ASSAY
AZF System Y-chromosome is a qualitative tests that allow the detection by Real Time PCR based on the amplification of the genome specific region using specific primers. In Real Time PCR the amplified product is detected using fluorescent dyes. These dyes are linked to oligonucleotide probes that bind specifically to the amplified product. The real-time monitoring of the fluorescence intensities during the reaction allows the detection of accumulating product without re-opening of the reaction tubes after the PCR run.
In principle, the analysis of only one non-polymorphic STS locus in each AZF region is sufficient to determine whether any STS deletion is present in AZFa, AZFb or AZFc. However, analyzing two STS loci in each region reinforces diagnostic accuracy, as deletions involve well-defined regions including many STS loci. Therefore, Sacace™ AZF System Y-chromosome kit was developed to detect two STS loci in each AZF region and choice of STS primers was based on the experience of many laboratories, the results of external quality control and the guidelines of American and European Andrology societies.
- These primers include:
- For AZFa: sY84, sY86
- For AZFb: sY127, sY134
- For AZFc: sY254, sY255
The Sacace™ AZF System Y-chromosome kit contains also a control primer pair that amplifies a unique region in both male and female DNA (ZFX/ZFY). These control primer pairs are internal controls for the multiplex amplification reactions and test the integrity of the genomic DNA sample. Finally, Sacace™ AZF System Y-chromosome kit also includes a primer pair that amplifies a region of the SRY gene, acting as control amplification for the testis-determining factor on the short arm of the Y chromosome and for the presence of Y-specific sequences when the ZFY gene is absent (e.g. in XX males).
The use of this primer set will enable the detection of almost all clinically relevant deletions and of over 95% of the deletions reported in the literature in the three AZF regions and is sufficient for routine diagnostics.
1 Review
-
higher quality
The quality of the product is very good and it was very effective and also no side effects were experienced.







