Description
Type II Collagen Detection Kit, Multi-Species - Cat Number: 6018 From Chondrex.
Research Field: ECM
Clonality: N/A
Cross-Reactivity:
Host Origin: N/A
Applications: N/A
Isotype: N/A
Detection Range: 200 µg/ml-3.13 µg/ml
Sample Type: Culture Media, Solubilized Collagen
Concentration: N/A
Immunogen:
PRODUCT SPECIFICATIONS
DESCRIPTION: ELISA kit to quantify multi-species Type II collagen
FORMAT: 96-well ELISA plate with removeable strips
ASSAY TYPE: Sandwich ELISA
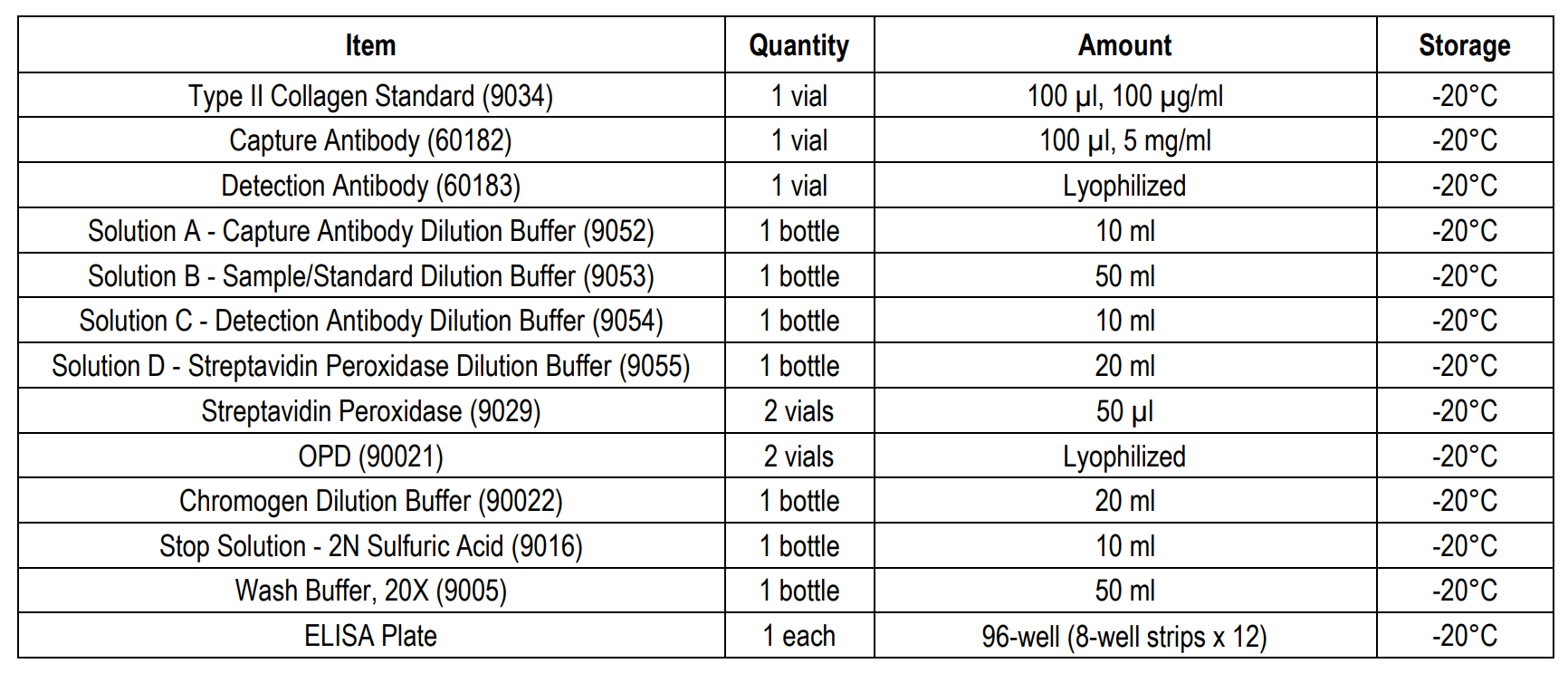
ASSAY TIME: 3.5 hours (1-step protocol) 5.5 hours (2-step protocol)
STANDARD RANGE: 200 ng/ml - 3.1 ng/ml
NUMBER OF SAMPLES: Up to 40 (duplicate) samples/plate
SAMPLE TYPES: Solubilized collagen and cell culture media
RECOMMENDED SAMPLE DILUTIONS: 1:1 - 1:1000
CHROMOGEN: OPD (read at 490 nm)
STORAGE: -20°C for 12 months
VALIDATION DATA: Intra-assay (2.1-4.4%)/Inter-assay (6.3-9.3%)/Spiking Test (86-97%)
NOTES: This kit has the option to run the standard and detection antibody steps in either a 1-step or 2-step procedure.
INTRODUCTION
Type II collagen is unique among the collagen family, and its tissue distribution is limited to avascular tissues such as cartilage and the vitreous body of the eye. As type II collagen can induce arthritis in experimental animals, autoimmunity to type II collagen is suspected in the pathogenesis of certain autoimmune diseases in humans such as rheumatoid arthritis (RA), eye diseases associated with RA, and relapsing polychondritis, which affects specific tissues containing type II collagen. The Type II Collagen Detection ELISA Kit (Cat # 6018) is designed to quantify the amount of solubilized native type II collagen from various species such as human, monkey, porcine, bovine, rat, mouse, rabbit, equine, dog, and chick in cell culture samples or tissue specimens by ELISA (Table 2). This kit has the option to run the standard and detection antibody steps in a 1- or 2-step procedure (Assay Outline). Chondrex, Inc. recommends running the 1-step protocol to save time. However, the 2-step assay protocol follows the same protocol as that of Chondrex, Inc's Type I Collagen Detection ELISA kits and both protocols yield comparable standard curves and identical sample results for this kit (Figure 1). Please use the appropriate protocol best
suited for your studies.
KIT COMPONENTS
ASSAY OUTLINE
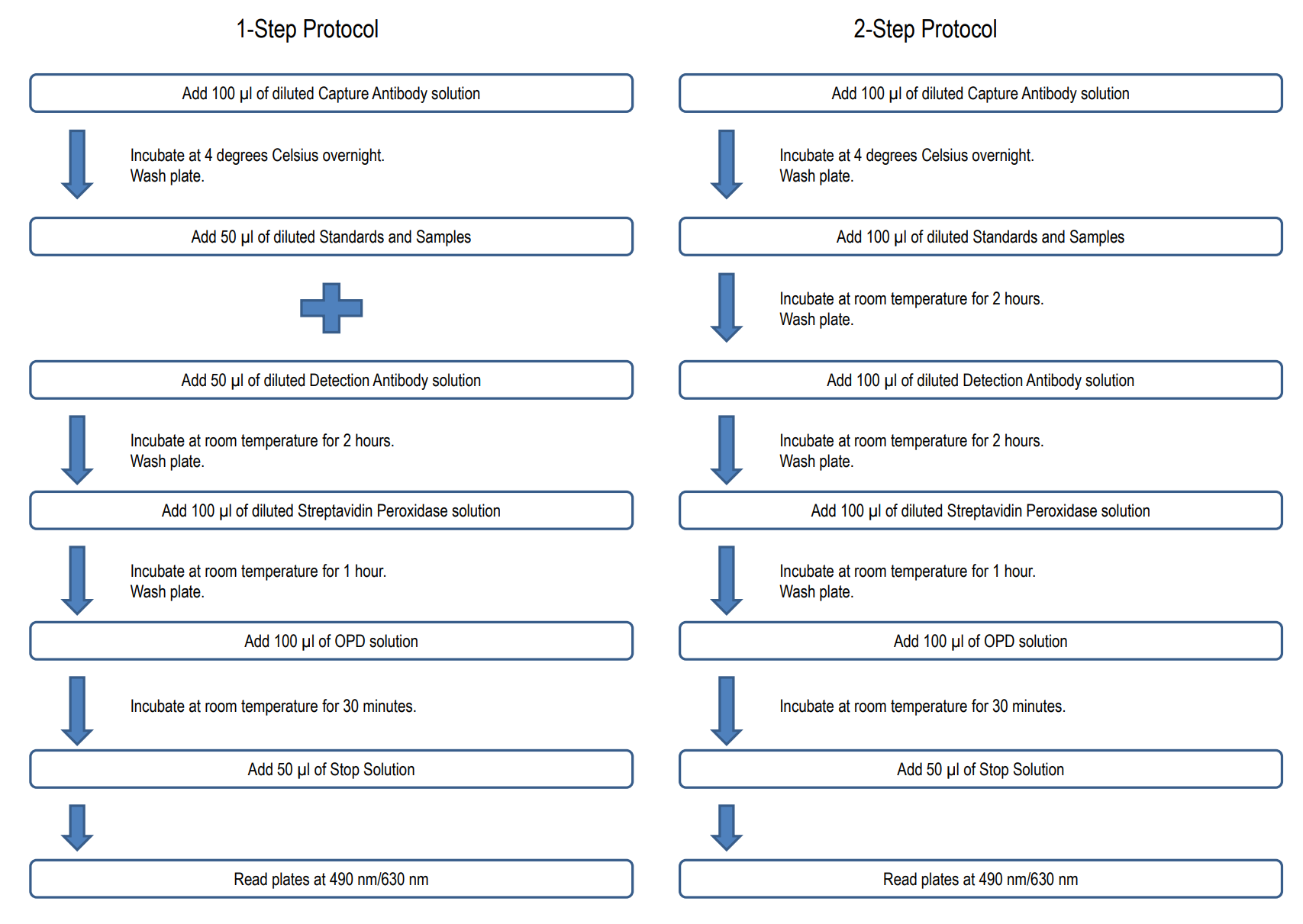
PLATE MAPPING
SOLUBILIZING COLLAGEN
For determining collagen content in cultured cell layers and tissues by ELISA, solubilizing collagen is required. For tissue specimens, a limited digestion with pepsin is highly recommended for native collagen preparation as other neutral proteinases, such as pronase and papain, digest collagen into small peptides. Pepsin only digests telopeptides located on both the N- and C-terminals of the collagen molecule and is not capable of digesting the helical conformation region of the collagen molecule itself. Please inquire with Chondrex, Inc. customer service for “Tips on Solubilization of Collagen”.
Solubilizing collagen from tissues by limited pepsin digestion (generally collagen to pepsin ratio is 100:1) depends on the types of tissues and the contents of the intra- and inter-molecular cross-linkages. For example, bone and Achilles tendon are resistant to pepsin digestion and only 10-20% of the collagen tissue will be solubilized. Young calf skin collagen will be completely solubilized by pepsin digestion within 24-48 hours, while it takes 7-9 days to solubilize adult skin. Pepsin resistant insoluble collagen might be solubilized by alkaline treatment.
Suspend insoluble collagen in cold 0.1N NaOH solution containing 10% Na2SO4 and 0.1M Amine such as Tris and incubate at 4°C for 1-2 weeks. After treatment of collagen with alkaline, neutralize the pH to 5.0 with HCl, and then dilute it with 0.05M acetic acid or neutral buffer such as 0.1M Tris-0.3M NaCl, pH 7.5.
Therefore, the optimum solubilization condition for individual samples should be determined before processing samples. Collagen can be analyzed by 6% SDS-gel under non-reducing conditions, using authentic type I collagen as a standard. If samples contain bands larger than the gamma-chain (MW = 300 Kd), the samples must be further digested by pepsin or elastase which will digest the intra- and inter-collagen cross-linkages. On the other hand, if smaller bands or smear bands are observed under the α-chain (MW = 100 Kd), the samples might be over-digested. Therefore, it is critical to understand the biological and physico-chemical properties of individual collagen samples.
NOTES BEFORE USING ASSAY
NOTE 1: It is recommended that the standard and samples be run in duplicate.
NOTE 2: Warm up all buffers to room temperature before use.
NOTE 3: Crystals may form in Wash Buffer, 20X when stored at cold temperatures. If crystals have formed, warm the wash buffer by placing the bottle in warm water until crystals are completely dissolved.
NOTE 4: Measure exact volume of buffers using a serological pipet, as extra buffer is provided.
NOTE 5: Cover the plate with plastic wrap or a plate sealer after each step to prevent evaporation from the outside wells of the plate.
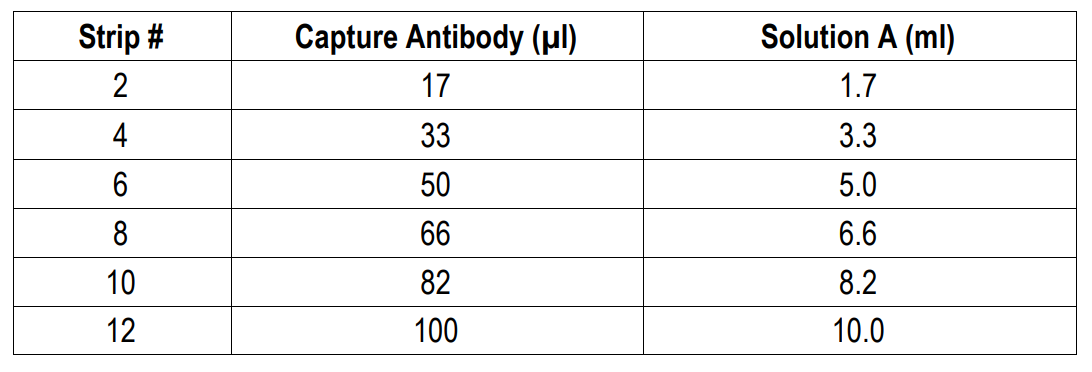
NOTE 6: For partial reagent use, please see the assay protocol’s corresponding step for the appropriate dilution ratio. For example, if the protocol dilutes 50 µl of a stock solution in 10 ml of buffer for 12 strips, then for 6 strips, dilute 25 µl of the stock solution in 5 ml of buffer. Partially used stock reagents may be kept in their original vials and stored at -20⁰C for use in a future assay.
NOTE 7: This kit contains animal components from non-infectious animals and should be treated as potential biohazards in use and for disposal.
1-STEP ASSAY PROCEDURE
1. Add Capture Antibody: Dilute one vial of Capture Antibody with 10 ml of Capture Antibody Dilution Buffer (Solution A). Alternatively, dilute according to assay needs. Add 100 µl of capture antibody solution to each well and incubate at 4°C overnight. Any remaining Capture Antibody Stock Solution can be stored at -20°C for future use.
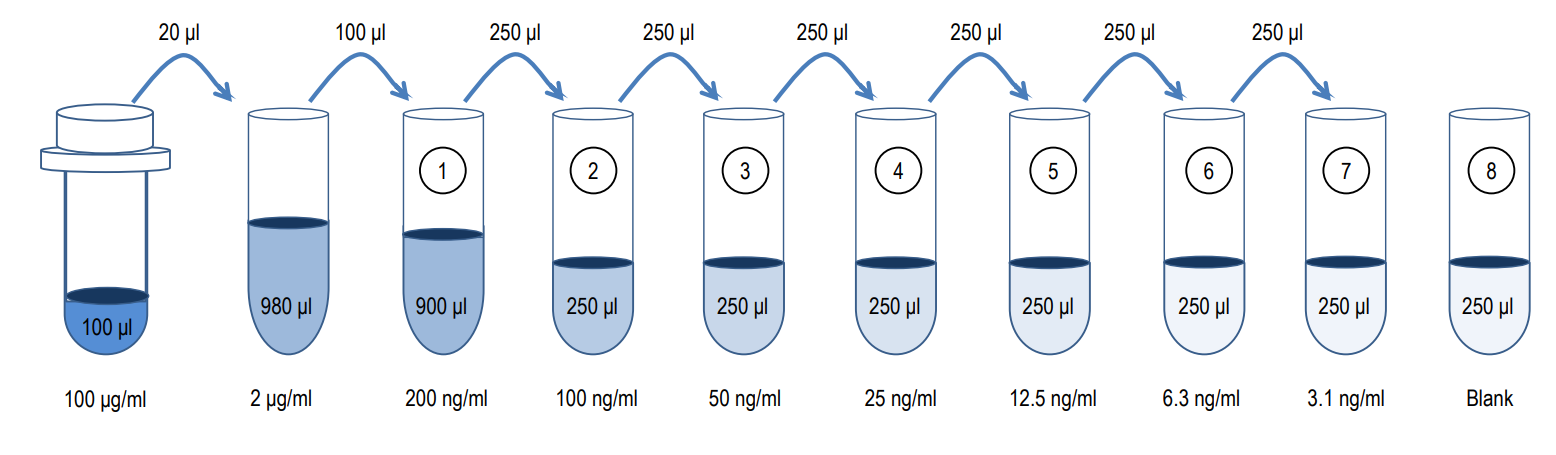
2. Prepare Standard Dilutions: The standard range is 3.1-200 ng/ml. Prepare serial dilutions of the standard by mixing 20 µl of 100 µg/ml standard with 980 µl of Sample/Standard Dilution Buffer (Solution B) to make a 2,000 ng/ml stock solution. Mix 100 µl of the 2,000 ng/ml stock solution with 900 µl of Solution B to make a 200 ng/ml standard stock solution. Then serially dilute it with Solution B.
For example, mix 250 µl of the 200 ng/ml standard stock solution with an equal volume of Solution B to make a 100 ng/ml standard stock solution and repeat it five more times for 50, 25, 12.5, 6.3, and 3.1 ng/ml standard stock solutions. The original standard stock solution may be stored at -20⁰C for use in a future assay. Chondrex, Inc. recommends making fresh serial dilutions for each assay.
3. Prepare Sample Dilutions: Dilute tissue samples 1:1-1:1000 with Solution B depending on the estimated collagen content in the samples. The samples must be diluted with Solution B to maintain optimal assay conditions.
NOTE: Cell samples and culture media can be assayed at a 1:1 dilution with Solution B.
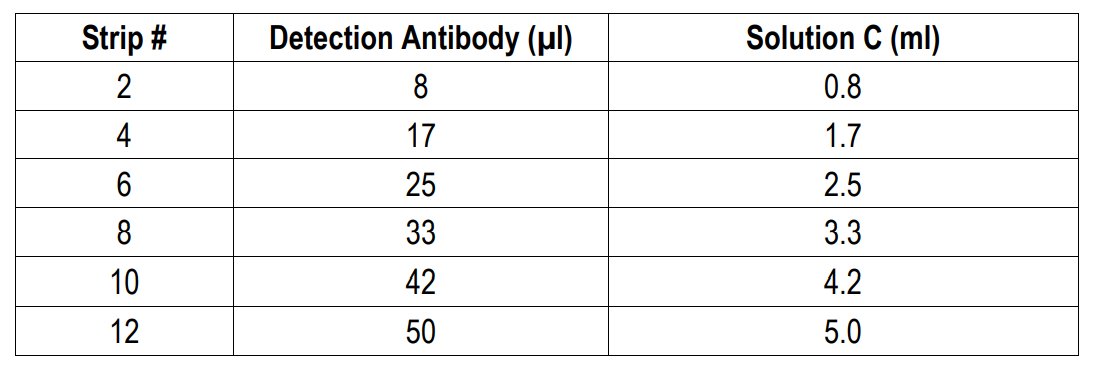
4. Prepare Detection Antibody: Dissolve one vial of Detection Antibody in 5 ml Detection Antibody Dilution Buffer (Solution C). Alternatively, dissolve one vial of Detection Antibody in 50 µl of Solution C and dilute accordingly.
5. Dilute Wash Buffer: Dilute 50 ml of 20X wash buffer in 950 ml of distilled water (1X wash buffer). Wash the plate with 1X wash buffer at least 3 times using a wash bottle with manifold or an automated plate washer. Empty the plate by inverting it and blotting on a paper towel to remove excess liquid. Do not allow the plate to dry out.
6. Add Standards, Samples, and Detection Antibodies: Add 50 µl of standards, Solution B (blank), and samples to appropriate wells in duplicate. Add 50 µl of detection antibody solution to all wells. Incubate at room temperature for 2 hours.
7. Wash: Wash the plate with 1X wash buffer at least 3 times using a wash bottle with manifold or an automated plate washer. Empty the plate by inverting it and blotting on a paper towel to remove excess liquid. Do not allow the plate to dry out.
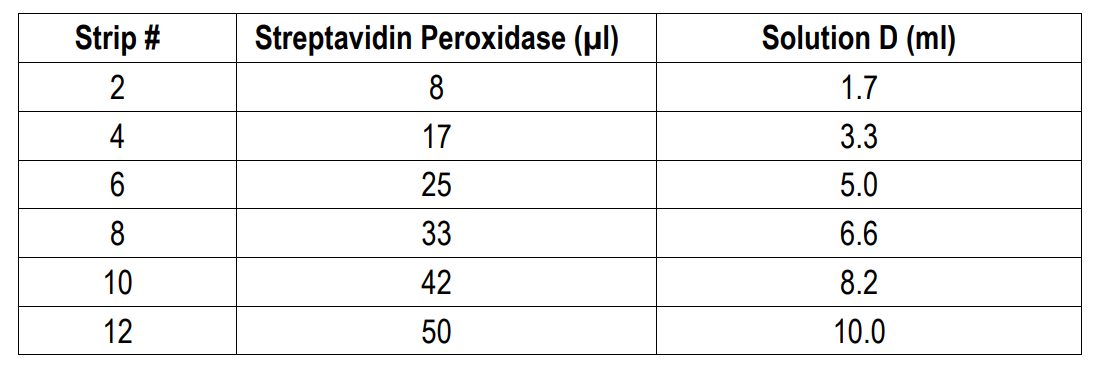
8. Add Streptavidin Peroxidase: Dilute one vial of Streptavidin Peroxidase in 10 ml of Streptavidin Dilution Buffer (Solution D). Add 100 µl of streptavidin peroxidase solution to each well and incubate at room temperature for 1 hour.
9. Wash: Wash the plate with 1X wash buffer at least 3 times using a wash bottle with manifold or an automated plate washer. Empty the plate by inverting it and blotting on a paper towel to remove excess liquid. Do not allow the plate to dry out.
10. Add OPD: Dissolve one vial of OPD with 10 ml of Chromogen Dilution Buffer just prior to use. Add 100 µl of OPD solution to each well immediately after washing the plate and incubate for 30 minutes at room temperature.
11. Stop: Add 50 µl of 2N sulfuric acid (Stop Solution) to each well.
12. Read Plate: Read the OD values at 490 nm. If the OD values of samples are greater than the OD values of the highest standard, re-assay the samples at a higher dilution. A 630 nm filter can be used as a reference.






