Description
The Chloramphenicol ELISA KIT is an immunoassay for the detection of Chloramphenicol in contaminated samples.
Antibiotic residues in foods pose a serious threat to public health. Human use of Chloramphenicol, a broad- spectrum antibiotic,
is found primarily in developing countries due to its low cost. The use of Chloramphenicol in developed nations is generally
limited to topical application for the treatment of eye infections; as chloramphenicol can adversely affect bone marrow, causing
aplastic anemia, which is usually fatal. Oral chloramphenicol treatment is therefore only considered appropriate for the treatment
of MRSA or other highly antibiotic resistant infections. Chloramphenicol is more frequently used in the veterinary treatment of
infections in small mammals and also in amphibians to treat chytridiomycosis; a fungal disease responsible for the loss of onethird of all species of frogs within the past 30 years. The use of Chloramphenicol in food-producing animals is prohibited in many
countries including the United States, Canada, the European Union, and Australia due to the high potential risk of severe effects
such as aplastic anemia, allergic reactions, and the promotion of antibiotic resistance. The U.S., Canada, and the EU have also
imposed bans on all imported foods containing Chloramphenicol residues. The monitoring of water sources and food products,
such as meat, milk and honey, for antibiotic residues is necessary to ascertain that these compounds are not misused and do
not present a danger to human or animal health.
Principle Of The Test
The test is a direct competitive ELISA based on the recognition of Chloramphenicol by specific antibodies. Chloramphenicol,
when present in a sample, and a Chloramphenicol-enzyme conjugate compete for the binding sites of monoclonal mouse antiChloramphenicol antibodies in solution. The Chloramphenicol antibodies are then bound by a second antibody (goat anti-mouse)
immobilized on the plate. After a washing step and addition of the substrate solution, a color signal is produced. The intensity of
the blue color is inversely proportional to the concentration of Chloramphenicol present in the sample. The color reaction is
stopped after a specified time and the color is evaluated using an ELISA reader. The concentrations of the samples are
determined by interpolation using the standard curve constructed with each run
Reagents And Materials Provided
1. Microtiter plate coated with a second antibody (goat anti-mouse).
2. Chloramphenicol Standards : 0, 0.025, 0.050, 0.1, 0.25, 0.5, 1.0, and 2.0 ng/mL.
3. Monoclonal mouse anti-Chloramphenicol Antibody, 3 vials (lyophilized). 3.5mL/vial after reconstitution.
4. Antibody Diluent, 15mL.
5. Chloramphenicol-HRP Conjugate, 3 vials (lyophilized). 3.5 mL/vial after reconstitution.
6. Conjugate Diluent, 15 mL.
7. Sample Diluent concentrate (10X), 25 mL, must be diluted before use. Use to dilute samples.
8. Wash Solution (5X) Concentrate, 100 mL.
9. Color (Substrate) Solution (TMB), 16 mL.
10. Stop Solution, 12 mL.
Materials Required But Not Supplied
1. Micro-pipettes with disposable plastic tips (10-200 and 200-1000 µL)
2. Multi-channel pipette (10-250 µL) or stepper pipette with plastic tips (10-250 µL)
3. Microtiter plate reader (wave length 450 nm)
4. Timer
5. Tape or Parafilm
Storage
The Chloramphenicol ELISA Kit should to be stored in the refrigerator (4–8°C). The solutions must be allowed to reach room
temperature (20-25°C) before use. Reagents may be used until the expiration date on the box. The antibody and conjugate are
supplied in lyophilized form (3 vials of each). Before each assay, the required volumes of lyophilized antibody and conjugate
must be reconstituted with the appropriate diluent. Reconstitute only the amount needed for the samples to be run, as the
reconstituted solutions will only remain viable for one week (store refrigerated).
Specimen Collection And Handling
Fish/Shrimp Extraction
1. Weigh 3g of homogenized fish or de-shelled shrimp (should have a paste-like consistency) into a 10mL or larger glass vial
with a Teflon-lined cap.
2. Add 6mL of Ethyl Acetate. Vortex thoroughly. Mix using an overhead tube rotator for 10 minutes.
3. Centrifuge vial for 10 minutes at 3000 g. Pipette 4 mL of the supernatant (top layer) into a clean vial.
4. Evaporate to dryness at 40-60ºC under a gentle stream of nitrogen.
5. Add 1 mL of Iso-octane / Trichloromethane (2:3) and vortex thoroughly to re-dissolve.
6. Add 2 mL of Sample Diluent (1X) and vortex thoroughly.
7. Centrifuge vial for 10 minutes at 4000 g.
8. Pipette supernatant (top layer) into a clean vial. This will then be analyzed as sample.
The ELISA result will show the Chloramphenicol concentration contained in the fish/shrimp samples (no correction factor is
necessary). Highly contaminated samples (those outside of the calibration range of the assay) must be diluted and re-analyzed.
Honey Sample Extraction
1. Add 3 g of honey to a clean glass vial with a Teflon-lined cap.
2. Add 3 mL of distilled or deionized water. Vortex.
3. Add 6 mL of Ethyl Acetate. Vortex. Mix using an overhead tube rotator for 10 minutes.
4. Centrifuge vial for 10 minutes at 3000 g. Pipette 4 mL of the supernatant (top layer) into a clean vial.
5. Evaporate to dryness at 40-60℃ under a gentle stream of nitrogen.
6. Add 1 mL of Iso-octane / Trichloromethane (2:3) and vortex thoroughly to re-dissolve.
7. Add 1 mL of Sample Diluent (1X) and vortex thoroughly. Centrifuge vial for 10 minutes at 3000 g.
8. Pipette supernatant into a clean vial. This will then be analyzed as sample.
The Chloramphenicol concentration contained in honey samples is then determined by dividing the ELISA result by the
concentration factor of 2. Highly contaminated samples (those outside of the calibration range of the assay) must be diluted and
re-analyzed.
Milk
No sample extraction is necessary for the analysis of milk samples. Highly contaminated samples (those outside of the
calibration range of the assay) will need to be diluted and re-analyzed.
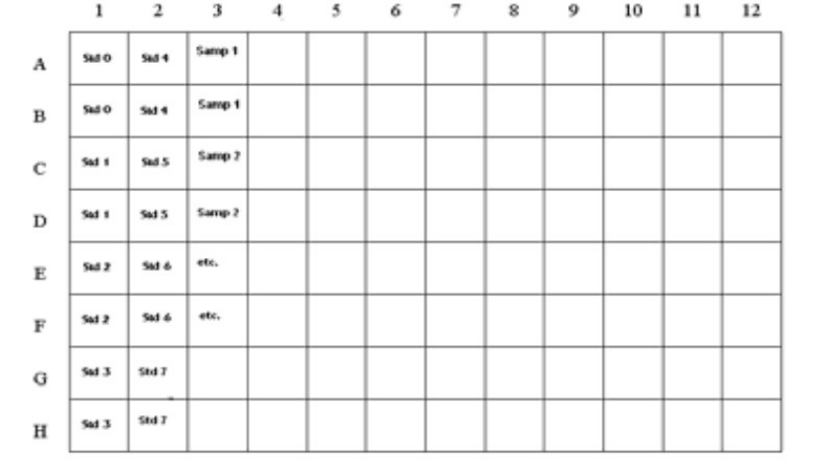
Plate Preparation
The microtiter plate consists of 12 strips of 8 wells, which can be used individually for the test. The standards must be run with
each test. Never use the values of standards which have been determined in a test performed previously.
Std 0-Std 7: Standards
0; 0.025; 0.050; 0.1; 0.25; 0.5; 1.0; 2.0 ppb
Samp1, Samp2, etc.: Samples
Figure 1:
Reagent Preparation
Micro-pipetting equipment and pipette tips for pipetting the standards and the samples are necessary. We recommend using a
multi-channel pipette or a stepping pipette for adding the conjugate, antibody, substrate and stop solutions in order to equalize
the incubations periods of the solutions on the entire microtiter plate. Please use only the reagents and standards from one
package lot in one test, as they have been adjusted in combination.
1. Adjust the microtiter plate and the reagents to room temperature before use.
2. Remove the number of microtiter plate strips required from the foil bag. The remaining strips are stored in the foil bag and ziplocked closed. Store the remaining kit in the refrigerator (4-8°C).
3. The standard solutions, substrate, and stop solutions are ready to use and do not require any further dilutions.
4. The antibody provided is lyophilized (3 vials). Before each assay, calculate the volume of antibody needed (when
reconstituted, each vial will provide enough antibody for approximately 65 wells). Reconstitute only the amount necessary for the
samples to be analyzed. Once reconstituted, the antibody solution will only remain viable for 1 week (store refrigerated). If
additional samples are to be analyzed greater than one week from reconstitution, a new vial of antibody will need to be
prepared. To reconstitute, add 3.5 mL of Antibody Diluent to each vial of antibody required and vortex thoroughly.
5. The conjugate provided is lyophilized (3 vials). Before each assay, calculate the volume of conjugate needed (when
reconstituted, each vial will provide enough conjugate for approximately 65 wells). Reconstitute only the amount necessary for
the samples to be analyzed. Once reconstituted, the conjugate solution will only remain viable for 1 week (store refrigerated). If
additional samples are to be analyzed greater than one week from reconstitution, a new vial of conjugate will need to be
prepared. To reconstitute, add 3.5 mL of Conjugate Diluent to each vial of conjugate required and vortex thoroughly.