Description
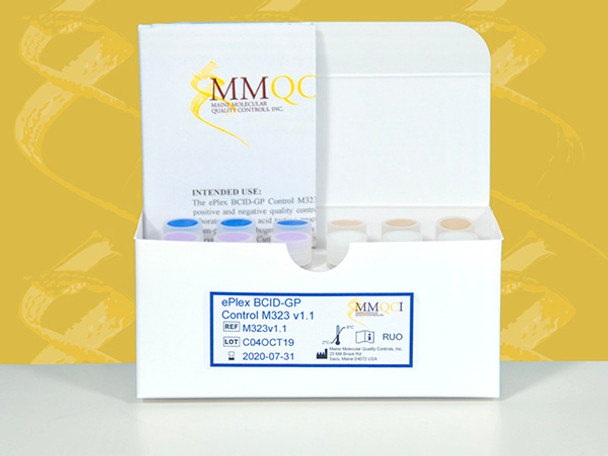
MMQCI ePlex BCID-GP Control Panel M323 v1.1
Part Number: M323v1.1
Kit Contains:
12 tubes x 50 μL
3 each Positive A, Positive B1, Positive C & Negative
ePlex BCID-GP Control M323 v1.1
INTENDED USE:
The ePlex BCID-GP Control M323 v1.1 is intended for use as an external positive and negative quality control to monitor the performance of in vitro laboratory nucleic acid testing procedures for the qualitative detection of the gram-positive pathogens: Bacillus cereus group, Bacillus subtilis group, Corynebacterium, Cutibacterium acnes (P.acnes), Enterococcus, Enterococcus faecalis, Enterococcus faecium, Lactobacillus, Listeria, Listeria monocytogenes, Micrococcus, Staphylococcus, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus lugdunensis, Streptococcus, Streptococcus agalactiae, Streptococcus anginosus group, Streptococcus pneumoniae, Streptococcus pyogenes; Pan targets: Pan Candida and Pan GramNegative; and resistance genes: mecA, mecC, vanA and vanB on the ePlex® Blood Culture Identification Gram-Positive (BCID-GP) Panel performed on the ePlex System (GenMark Diagnostics, Inc.). The ePlex BCID-GP Control M323 v1.1 is composed of synthetic DNA specifically designed for and intended to be used solely with the ePlex BCID-GP Panel assay. This product is not intended to replace manufacturer controls provided with the device.
PRODUCT SUMMARY and PRINCIPLE:
ePlex BCID-GP Control M323 v1.1 is composed of 4 controls, ePlex BCID-GP Positive A, ePlex BCID-GP Positive B1, ePlex BCID-GP Positive C, and ePlex BCID-GP Negative. The ePlex BCID-GP Control M323 v1.1 positive controls contain surrogate control material composed of synthetic DNA corresponding to genome segments of pathogens and resistance genes listed in Table 1. ePlex BCID-GP Negative contains no DNA. Routine use of quality controls that are consistent lot to lot assists the laboratory in identifying shifts, trends, and increased frequency of random errors caused by variations in the test system, such as failing reagents. Early investigation can prevent failed assay runs.
COMPOSITION:
ePlex BCID-GP Control M323 v1.1 is comprised of 12 tubes, 3 tubes of each positive control, A, B1, and C, and 3 tubes of negative control, 50μL each. The ePlex BCID-GP Positive controls contain synthetic DNA suspended in a noninfectious solution of buffers, preservatives and stabilizers. Table 1. lists the pathogens and resistance genes that are monitored by the ePlex BCID-GP Control M323 v1.1 when tested by the ePlex BCID-GP Panel on the ePlex System.
STORAGE and STABILITY:
ePlex BCID-GP Control M323 v1.1 should be stored refrigerated at 2-8°C. Unopened ePlex BCID-GP Control M323 v1.1 material is stable through the expiration date printed on the kit label when continuously stored refrigerated. ePlex BCID-GP Control M323 v1.1 components are for single use. Discard after use according to your local and federal regulations.
PRECAUTIONS and WARNINGS:
- Use the control as provided. Do not dilute or transfer to another tube.
- This product is intended for in vitro analytical testing and is provided for Research Use Only, not for use in diagnostic procedures.
- ePlex BCID-GP Control M323 v1.1 is only for use with ePlex BCID-GP Panel on the ePlex System. It does not contain the entire genome of the pathogens listed in Table 1.
- This product is not intended for use as a substitute for the internal controls provided in ePlex BCID-GP Panel.
- This product does not contain any biological material of human or animal origin. Universal Precautions are NOT required when handling this product.
- ePlex BCID-GP Control M323 v1.1 cannot be cloned, sold, or transferred without the explicit written consent of MMQCI.
- Quality control materials should be used in accordance with local, state, federal regulations and accreditation requirements.
INSTRUCTIONS FOR USE:
For complete ePlex BCID-GP Panel instructions, refer to the ePlex BCID-GP Panel package insert provided by GenMark Diagnostics, Inc.
- Allow the control to be tested to come completely to room temperature (18° – 25°C).
- Immediately before use thoroughly mix the control by flicking the tube several times, followed by vortexing for 5-10 seconds. Shake the tube down or tap it on bench to remove any droplets remaining in the cap.
- Use a calibrated pipette to aspirate 50µL of control and transfer into the cartridge sample port chamber.
- Continue to process and analyze the control with the ePlex BCID-GP Panel test on the ePlex System as you would a patient sample. Note: Sample can be pre-defined as External Control to generate a BCIDGP External Control Report. Refer to ePlex Operator Manual for more information on defining external controls on ePlex System.
- Discard controls after use according to your local and federal regulations.
EXPECTED VALUES:
Table 1. lists the expected results when the controls are tested with ePlex BCIDGP Panel on the ePlex System. The laboratory should follow Good Laboratory Practice (GLP) and establish its own performance characteristics for ePlex BCID-GP Control M323 v1.1 in demonstrating adequate system performance.
Table 1. Expected Results: ePlex BCID-GP Control M323 v1.1
|
Control Name |
ePlex BCID-GP Positive A |
ePlex BCID-GP Positive B1 |
ePlex BCID-GP Positive C |
ePlex BCID-GP Negative |
|
Part Number |
M32437 |
M33737 |
M33837 |
M50237 |
|
Gram-Positive Pathogen |
Result |
|||
|
Bacillus cereus group |
Detected |
Not Detected |
Not Detected |
Not Detected |
|
Bacillus subtilis group |
Detected |
Not Detected |
Not Detected |
Not Detected |
|
Corynebacterium |
Detected |
Not Detected |
Not Detected |
Not Detected |
|
Cutibacterium acnes (P. acnes) |
Not Detected |
Detected |
Not Detected |
Not Detected |
|
Enterococcus |
Detected |
Not Detected |
Not Detected |
Not Detected |
|
Enterococcus faecalis |
Detected |
Not Detected |
Not Detected |
Not Detected |
|
Enterococcus faecium |
Detected |
Not Detected |
Not Detected |
Not Detected |
|
Lactobacillus |
Not Detected |
Detected |
Not Detected |
Not Detected |
|
Listeria |
Not Detected |
Detected |
Detected |
Not Detected |
|
Listeria monocytogenes |
Not Detected |
Not Detected |
Detected |
Not Detected |
|
Micrococcus |
Not Detected |
Not Detected |
Detected |
Not Detected |
|
Staphylococcus |
Not Detected |
Detected |
Detected |
Not Detected |
|
Staphylococcus aureus |
Not Detected |
Detected |
Not Detected |
Not Detected |
|
Staphylococcus epidermidis |
Not Detected |
Not Detected |
Detected |
Not Detected |
|
Staphylococcus lugdunensis |
Not Detected |
Not Detected |
Detected |
Not Detected |
|
Streptococcus |
Detected |
Not Detected |
Not Detected |
Not Detected |
|
Streptococcus agalactiae |
Detected |
Not Detected |
Not Detected |
Not Detected |
|
Streptococcus anginosus group |
Detected |
Not Detected |
Not Detected |
Not Detected |
|
Streptococcus pneumoniae |
Detected |
Not Detected |
Not Detected |
Not Detected |
|
Streptococcus pyogenes |
Detected |
Not Detected |
Not Detected |
Not Detected |
|
Pan Targets |
Result |
|||
|
Pan Candida |
Not Detected |
Detected |
Not Detected |
Not Detected |
|
Pan Gram-Negative |
Not Detected |
Not Detected |
Detected |
Not Detected |
|
Resistance Gene |
Result |
|||
|
mecA |
N/A |
Not Detected |
Detected |
N/A |
|
mecC |
N/A |
Detected |
Not Detected |
N/A |
|
vanA |
Detected |
N/A |
N/A |
N/A |
|
vanB |
Detected |
N/A |
N/A |
N/A |
Notice: ePlex is a registered trademark of GenMark Diagnostics, Inc.
2 Reviews
-
perfect
The ePlex BCID-GP Control M323 v1.1 is intended for use as an external positive and negative quality control to monitor the performance of in vitro laboratory nucleic acid testing procedures for the qualitative detection of gram-positive pathogens. all the staff, their efficient working and skilled qualities are totally excellent
-
ePlex BCID-GP Control M323 v1.1
The ePlex BCID-GP Control M323 v1.1 is intended for use as an external positive and negative quality control to monitor the performance of in vitro laboratory nucleic acid testing procedures for the qualitative detection of the gram-positive pathogens: Bacillus cereus group, Bacillus subtilis group, Corynebacterium, Cutibacterium acnes (P.acnes), Enterococcus, Enterococcus faecalis, Enterococcus faecium, Lactobacillus, Listeria, Listeria monocytogenes, Micrococcus, Staphylococcus, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus lugdunensis, Streptococcus, Streptococcus agalactiae, Streptococcus anginosus group, Streptococcus pneumoniae, Streptococcus pyogenes; Pan targets: Pan Candida and Pan Gram-Negative; and resistance genes: mecA, mecC, vanA and vanB on the ePlex® Blood Culture Identification Gram-Positive (BCID-GP) Panel performed on the ePlex System (GenMark Diagnostics, Inc.). The ePlex BCID-GP Control M323 v1.1 is composed of synthetic DNA specifically designed for and intended to be used solely with the ePlex BCID-GP Panel assay. This product is not intended to replace manufacturer controls provided with the device.